Which statement does not support the endosymbiotic theory? This question probes the very foundation of eukaryotic cell evolution, a theory explaining the origin of mitochondria and chloroplasts. The endosymbiotic theory posits that these organelles were once free-living prokaryotes that established a symbiotic relationship with a host cell, eventually becoming integrated into the eukaryotic cellular structure. However, certain observations and findings have challenged aspects of this theory, leading to ongoing scientific debate and refinement of our understanding of eukaryotic origins.
This exploration delves into the evidence supporting and contradicting the endosymbiotic theory, focusing on specific discrepancies and their implications for our understanding of cellular evolution.
The provided Artikel details extensive comparative analyses of mitochondrial and chloroplast structures, functions, and genetic material with their prokaryotic counterparts. These comparisons encompass ribosomal structure and function, genome size and gene content, membrane composition, metabolic pathways, and evolutionary relationships. By examining the similarities and, crucially, the differences, we can identify areas where the endosymbiotic theory finds strong support and areas where it faces challenges.
This detailed examination aims to provide a comprehensive understanding of the current state of knowledge regarding the endosymbiotic theory and its limitations.
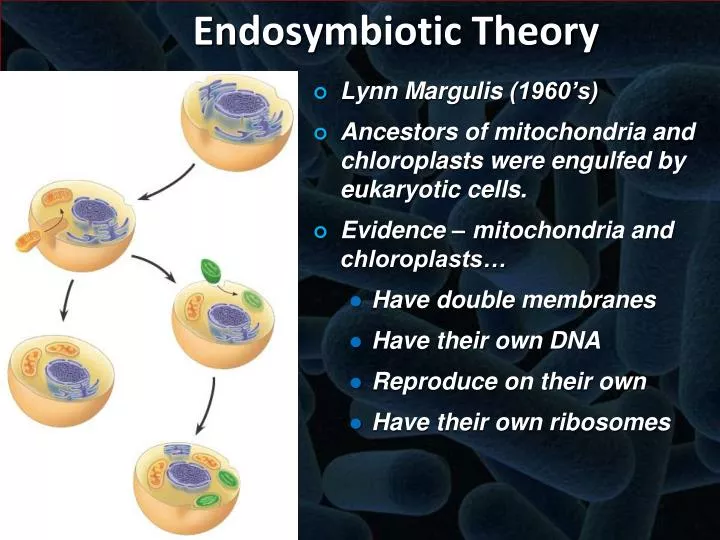
Mitochondrial Ribosomes
The intricate dance of life within our cells reveals a profound mystery: the existence of mitochondria, the powerhouses that fuel our being. These organelles, with their own unique genetic material and protein synthesis machinery, hold clues to the very origins of eukaryotic life. A key component of this ancient story lies within the structure and function of mitochondrial ribosomes, tiny molecular machines that build the proteins essential for mitochondrial respiration and energy production.
Their unique characteristics offer a window into the evolutionary journey that shaped life as we know it.
Comparative Analysis of Mitochondrial and Cytoplasmic Ribosomes
The following table details the structural differences between mitochondrial and cytoplasmic ribosomes, highlighting the profound implications for protein synthesis. These variations reflect an evolutionary journey distinct from the cytoplasmic machinery, reflecting their prokaryotic ancestry.
| Feature | Mitochondrial Ribosomes (Mammalian) | Cytoplasmic Ribosomes (Mammalian) | Prokaryotic Ribosomes (E. coli) |
|---|---|---|---|
| rRNA Size and Type | 16S, 12S rRNA | 18S, 28S, 5.8S rRNA | 16S, 23S, 5S rRNA |
| Ribosomal Protein Composition | ~70 proteins | ~80 proteins | ~55 proteins |
| Sedimentation Coefficient (Svedberg units) | 55S | 80S | 70S |
| Chloramphenicol Sensitivity | Yes | No | Yes |
| Erythromycin Sensitivity | Yes | No | Yes |
| Cycloheximide Sensitivity | No | Yes | No |
| Overall Size and Shape | Smaller and less complex than cytoplasmic ribosomes | Larger and more complex | Similar in size to mitochondrial ribosomes |
The smaller size and distinct protein composition of mitochondrial ribosomes directly impact their efficiency and sensitivity to antibiotics. The sensitivity to chloramphenicol and erythromycin, antibiotics that target bacterial ribosomes, further underscores the evolutionary link between mitochondrial and prokaryotic ribosomes. Conversely, the insensitivity to cycloheximide, which inhibits eukaryotic cytoplasmic ribosomes, highlights the unique nature of mitochondrial protein synthesis. These differences contribute to the distinct regulation and control of mitochondrial protein production, essential for maintaining cellular energy homeostasis.
Mitochondrial and Cytoplasmic Protein Synthesis Pathways
The following flowchart visually represents the key steps in mitochondrial and cytoplasmic protein synthesis, emphasizing the unique aspects of each pathway. The differences observed reflect the evolutionary divergence of these two systems.[Illustrative Flowchart: A visual representation would show two parallel pathways, one for mitochondrial and one for cytoplasmic protein synthesis. Each pathway would detail the steps of initiation (including initiation factors), elongation (including elongation factors and tRNA charging), and termination (including termination factors).
The flowchart would highlight the differences in the specific factors and molecules involved in each pathway. For example, the mitochondrial pathway would use mitochondrial-specific initiation, elongation, and termination factors, while the cytoplasmic pathway would use its own set of factors. The flowchart would also show the distinct ribosomes involved in each pathway (55S for mitochondrial and 80S for cytoplasmic).
The involvement of mRNA would be common to both but the processing and translation mechanisms would differ.]The comparative analysis reveals that while both pathways share the fundamental principles of protein synthesis, they differ significantly in their components and regulatory mechanisms. The use of distinct initiation, elongation, and termination factors in each pathway allows for independent regulation and control of protein synthesis in the two cellular compartments.
The distinct tRNA pools and ribosomal structures further contribute to the unique characteristics of each pathway. This independent regulation is crucial for maintaining cellular homeostasis and responding to changing energy demands.
Evolutionary Origins of Mitochondrial Ribosomes
The following simplified phylogenetic tree illustrates the evolutionary relationship between mitochondrial ribosomes and bacterial ribosomes. The close relationship strongly supports the endosymbiotic theory.[Illustrative Phylogenetic Tree: A simplified tree would show the branching of mitochondrial ribosomes from a bacterial ancestor, possibly within the Alphaproteobacteria group. The tree would demonstrate the close phylogenetic relationship between mitochondrial ribosomes and bacterial ribosomes, supporting the endosymbiotic theory.
The tree should include relevant bacterial lineages and show the divergence point.]The evidence strongly supports the endosymbiotic hypothesis for the origin of mitochondrial ribosomes. The close similarity between mitochondrial and bacterial ribosomes in terms of rRNA sequences, protein composition, and antibiotic sensitivity provides compelling evidence for their shared ancestry. While horizontal gene transfer may have played a role in the evolution of mitochondrial ribosomes, the overall phylogenetic data overwhelmingly supports the endosymbiotic origin.
The non-endosymbiotic hypothesis lacks the robust supporting evidence provided by the striking similarities between mitochondrial and bacterial ribosomes.The role of specific ribosomal proteins in facilitating the adaptation of ribosomes to the mitochondrial environment remains an open question. For example, investigation into the specific mutations or modifications in ribosomal proteins that allowed for optimal function within the mitochondrial matrix could provide further insight into this evolutionary transition.
Antibiotic Sensitivity and Implications
The following table details the effects of various antibiotics on mitochondrial and cytoplasmic protein synthesis. The differential effects highlight the potential for selective targeting of mitochondrial functions.
| Antibiotic | Ribosomal Target (if known) | Effect on Mitochondrial Protein Synthesis | Effect on Cytoplasmic Protein Synthesis |
|---|---|---|---|
| Chloramphenicol | 50S ribosomal subunit | Inhibition | Minimal to no effect |
| Erythromycin | 50S ribosomal subunit | Inhibition | Minimal to no effect |
| Tetracycline | 30S ribosomal subunit | Inhibition | Inhibition |
| Cycloheximide | 60S ribosomal subunit | Minimal to no effect | Inhibition |
The differential sensitivity of mitochondrial and cytoplasmic ribosomes to various antibiotics provides a potential therapeutic window for targeting mitochondrial dysfunction in specific diseases without significantly affecting cytoplasmic protein synthesis.
Disease Relevance of Mitochondrial Ribosome Dysfunction
Mitochondrial ribosome dysfunction is implicated in a range of human diseases, often manifesting as mitochondrial myopathies, affecting muscle function, and encephalopathies, affecting brain function. Mutations in mitochondrial ribosomal proteins or rRNA genes can lead to impaired mitochondrial protein synthesis, resulting in reduced ATP production and cellular dysfunction. Examples include mitochondrial myopathies and Leigh syndrome.
Future Research Directions
Further research is needed to fully understand the complexities of mitochondrial ribosomes and their role in health and disease. Key areas include:
- A more comprehensive understanding of the regulatory mechanisms controlling mitochondrial protein synthesis.
- Detailed structural analysis of mitochondrial ribosomes using advanced imaging techniques.
- Development of novel therapeutic strategies targeting mitochondrial ribosomes for the treatment of mitochondrial diseases.
- Investigation into the role of mitochondrial ribosomes in aging and age-related diseases.
- Exploration of the evolutionary dynamics of mitochondrial ribosomes across different species.
Chloroplast DNA
The chloroplast, that verdant engine of life, holds within its very being a testament to the profound interconnectedness of all existence. Its DNA, a miniature echo of the ancient past, whispers secrets of symbiotic union and evolutionary dance, a story etched in the very molecules that sustain our world. Examining this DNA reveals both profound similarities and intriguing discrepancies that illuminate the ongoing debate surrounding the endosymbiotic theory.Chloroplast DNA (cpDNA) stands in fascinating contrast to nuclear DNA (nDNA), the genetic blueprint housed within the cell’s nucleus.
While both are double-stranded, circular molecules, their size and gene content differ significantly. cpDNA is considerably smaller than nDNA, typically ranging from 120 to 160 kilobases, a stark contrast to the vast gigabases found in nuclear genomes. This size difference reflects a reduction in genetic material over evolutionary time, a consequence of the transfer of genes from the chloroplast to the nucleus.
This transfer itself is a key piece of evidence supporting the endosymbiotic theory; however, the presence of some genes exclusively within the cpDNA, which are strikingly similar to their bacterial counterparts, presents a challenge to this narrative. The retention of these specific genes within the chloroplast suggests functional constraints, potentially reflecting irreducible complexity in certain metabolic pathways. This apparent stubbornness of certain genes to migrate into the nucleus hints at a deeper, more intricate relationship than a simple endosymbiotic takeover.
Chloroplast DNA Replication and Repair Mechanisms
Chloroplast DNA replication is a complex process, closely mirroring the replication mechanisms observed in bacteria. It involves the initiation of replication at specific sites, the unwinding of the DNA double helix, and the synthesis of new strands using DNA polymerase. Interestingly, cpDNA replication is often coupled to the cell cycle and chloroplast division, ensuring that each daughter chloroplast receives a complete copy of the genome.
Repair mechanisms within the chloroplast are also reminiscent of bacterial systems, employing processes like homologous recombination and nucleotide excision repair to correct DNA damage. These mechanisms are crucial for maintaining the integrity of the cpDNA, protecting it from the damaging effects of UV radiation and other environmental stressors. The bacterial-like nature of these replication and repair processes provides further evidence for the endosymbiotic origin of chloroplasts, echoing the cellular machinery of their ancient prokaryotic ancestors.
However, the integration of these mechanisms within the eukaryotic cellular context reveals the complex evolutionary interplay that has shaped the modern chloroplast. The subtle differences between chloroplast and bacterial repair mechanisms, including the integration of eukaryotic repair proteins, speak to the ongoing adaptation and co-evolution of the chloroplast within its host cell.
Comparative Gene Content of Chloroplast, Bacterial, and Nuclear DNA
The following table compares the gene content of chloroplast DNA (cpDNA), bacterial DNA (bDNA), and nuclear DNA (nDNA), focusing on aspects relevant to the endosymbiotic theory. The striking similarities between cpDNA and bDNA, particularly in genes encoding components of the photosynthetic apparatus and other essential metabolic pathways, provide strong support for the endosymbiotic hypothesis. However, the presence of genes in the nuclear DNA that were originally located in the chloroplast genome necessitates a more nuanced interpretation.
The transfer of genes to the nucleus highlights the integration of the chloroplast into the eukaryotic cell and the subsequent specialization of function.
| Gene Category | cpDNA | bDNA | nDNA | Relevance to Endosymbiotic Theory |
|---|---|---|---|---|
| Photosynthesis | Many genes present (e.g., rbcL, psbA) | Many homologous genes present | Some genes transferred, others remain in cpDNA | Strong evidence for endosymbiotic origin; gene transfer indicates integration. |
| Transcription/Translation | Some genes present (e.g., rRNA, tRNA) | Many homologous genes present | Most genes transferred | Evidence for endosymbiotic origin; extensive gene transfer reflects integration. |
| Metabolic Enzymes | Some genes present | Many homologous genes present | Many genes transferred | Supports endosymbiotic origin; gene transfer shows functional integration. |
| DNA Replication/Repair | Some genes present | Homologous genes present | Some genes transferred, some remain in cpDNA | Suggests partial integration; retention of some genes points to functional constraints. |
Mitochondrial Membrane Structure
The mitochondrial membrane, a marvel of cellular engineering, holds the key to understanding the powerhouse of the cell and its profound implications for the endosymbiotic theory. Its intricate architecture, a testament to billions of years of evolutionary refinement, reflects a story woven into the very fabric of life itself. By examining its structure and composition, we can gain deeper insights into the origins of this crucial organelle.
Mitochondrial Membrane Diagram
Imagine a double-walled sphere, the mitochondrion. The outer membrane, relatively smooth and permeable, encloses the entire organelle. Within, lies the intermembrane space, a crucial region for electrochemical gradients. The inner membrane, however, is a breathtaking landscape of infoldings called cristae, dramatically increasing its surface area. These cristae are not merely wrinkles; they are precisely organized structures housing the electron transport chain complexes (Complexes I-IV) and ATP synthase, the molecular machinery responsible for ATP synthesis – the cell’s primary energy currency.
Embedded within the matrix, the innermost compartment, are mitochondrial DNA, ribosomes, and various metabolic enzymes. The inner membrane is significantly less permeable than the outer membrane, maintaining the crucial proton gradient necessary for ATP production. A scale bar of 100nm would appropriately represent the relative sizes of these structures. The fluidity of the outer membrane is greater than the inner membrane due to differences in lipid composition.
Endosymbiotic Theory Support/Contradiction (Diagram Annotations)
(Note: Since I cannot create a visual diagram here, I will describe annotations. Imagine the previous diagram. )* Supporting Evidence (Green Annotations): The double membrane structure itself is a primary piece of supporting evidence. The inner membrane, with its bacterial-like lipid and protein composition (as discussed later), is annotated in green, highlighting its resemblance to a prokaryotic cell membrane. The presence of the intermembrane space, analogous to the periplasmic space in bacteria, is also annotated in green.
The presence of cristae, increasing surface area for ATP production, would be marked in green, reflecting a specialization consistent with the evolution of an endosymbiont.* Contradicting Evidence (Red Annotations): While the double membrane strongly supports the theory, certain aspects of the inner membrane’s lipid composition (e.g., the high cardiolipin content, a lipid not commonly found in bacteria at such high concentrations) would be annotated in red.
Differences in protein composition between the inner mitochondrial membrane and bacterial membranes, specifically those involved in protein import and membrane biogenesis, would also be highlighted in red, as this represents a departure from a purely bacterial-like structure. The highly integrated nature of the mitochondrial inner membrane with the host cell’s machinery, particularly regarding protein import mechanisms, would be annotated in red to reflect a departure from a completely independent prokaryotic ancestor.
Evolutionary Origin Evidence
- Evidence: The presence of a circular mitochondrial genome.
- Methodology: Electron microscopy and DNA sequencing.
- Endosymbiotic Theory Support/Challenge: This strongly supports the theory, as bacterial genomes are also typically circular. The sequence similarity between mitochondrial DNA and bacterial DNA further strengthens this support.
- Evidence: Mitochondrial ribosomes resemble bacterial ribosomes.
- Methodology: Biochemical analysis of ribosomal RNA and protein composition.
- Endosymbiotic Theory Support/Challenge: The structural and functional similarities between mitochondrial and bacterial ribosomes strongly support the endosymbiotic hypothesis, suggesting a common ancestry.
- Evidence: The double membrane structure itself.
- Methodology: Electron microscopy and biochemical analysis of membrane composition.
- Endosymbiotic Theory Support/Challenge: The inner membrane’s similarity to bacterial membranes, and the presence of an intermembrane space, are consistent with the engulfment of a bacterium by a host cell. However, some discrepancies in lipid composition (as discussed below) represent potential challenges to a straightforward interpretation.
Membrane Composition Comparison
| Component | Mitochondrial Inner Membrane | Mitochondrial Outer Membrane | Bacterial Membrane (E. coli) | Discrepancy & Endosymbiotic Theory Implications |
|---|---|---|---|---|
| Phospholipid Types | Phosphatidylcholine, phosphatidylethanolamine, cardiolipin | Phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine | Phosphatidylethanolamine, phosphatidylglycerol | Higher cardiolipin in inner mitochondrial membrane; differences in other phospholipids. High cardiolipin content is a key discrepancy, though the significance is debated. |
| Phospholipid Ratio | Variable, depending on species and metabolic state | Variable, depending on species and metabolic state | Variable, depending on species and growth conditions | Differences in ratios between mitochondrial and bacterial membranes exist, although the significance in refuting the theory is limited. |
| Cardiolipin Content | High | Low | Low | The significantly higher cardiolipin content in the inner mitochondrial membrane is a major point of discussion. |
| Protein Types | Electron transport chain complexes, ATP synthase, transporters | Porins, enzymes involved in lipid metabolism | Diverse, depending on species and function | Significant overlap but also unique proteins in each. The unique protein sets reflect adaptation and integration into the eukaryotic cellular machinery. |
| Protein Abundance | High | Lower than inner membrane | High | The high protein density in both membranes reflects their functional roles. |
Refuting Endosymbiotic Theory (Table)
| Discrepancy | Explanation | Refutation of Endosymbiotic Theory Aspect |
|---|---|---|
| High cardiolipin content in inner mitochondrial membrane | Cardiolipin is less abundant in most bacteria than in the inner mitochondrial membrane. | Challenges the direct evolutionary link to a specific bacterial ancestor; suggests later modifications. |
| Unique protein composition of mitochondrial membranes | Many proteins are unique to mitochondrial membranes and not found in bacteria. | Suggests extensive evolutionary adaptation and integration into the eukaryotic cellular machinery after the endosymbiotic event. |
| Mitochondrial protein import machinery | Complex systems for importing proteins into mitochondria are not found in bacteria. | Indicates extensive integration and co-evolution with the host cell, going beyond a simple symbiotic relationship. |
Written Summary
Analysis of mitochondrial membrane structure provides strong, albeit not unequivocal, support for the endosymbiotic theory. The double membrane structure, reminiscent of a prokaryotic cell engulfed by a host, along with the presence of a bacterial-like genome and ribosomes, strongly suggests an endosymbiotic origin. However, discrepancies exist, notably the high cardiolipin content in the inner mitochondrial membrane and the unique protein composition of both membranes.
These differences highlight the extensive evolutionary adaptation and integration of the mitochondrion into the eukaryotic cell, indicating a complex evolutionary history that extends beyond a simple engulfment event. Further research focusing on the evolutionary origins of specific mitochondrial proteins and lipids, as well as comparative genomics of diverse mitochondrial lineages, will continue to refine our understanding of this fascinating organelle’s history.
Antibiotic Sensitivity
The sensitivity of mitochondria to certain antibiotics offers a compelling lens through which to examine the endosymbiotic theory. This theory posits that mitochondria, the powerhouses of eukaryotic cells, originated from free-living bacteria that were engulfed by an ancestral eukaryotic cell. The remarkable susceptibility of mitochondria to antibiotics, particularly those targeting bacterial ribosomes, provides strong evidence supporting this ancient symbiotic relationship.
Examining the specific effects of these antibiotics, their mechanisms of resistance, and comparative effects on both mitochondria and bacteria allows for a deeper understanding of this pivotal evolutionary event.Mitochondrial Response to Ribosomal AntibioticsTetracycline, chloramphenicol, erythromycin, and streptomycin, all well-known inhibitors of bacterial protein synthesis, exert significant effects on mitochondrial protein synthesis. These antibiotics bind to the mitochondrial ribosome, hindering its function and ultimately reducing the rate of protein production.
The specific effects vary depending on the antibiotic and the concentration used. For example, tetracycline inhibits aminoacyl-tRNA binding to the A site of the ribosome, while chloramphenicol blocks peptidyl transferase activity. Erythromycin binds to the 50S ribosomal subunit, interfering with translocation, and streptomycin binds to the 30S subunit, causing misreading of mRNA. These actions lead to a reduction in the synthesis of various mitochondrial proteins crucial for oxidative phosphorylation and ATP production.
The extent of this reduction is concentration-dependent and can be quantified by measuring the incorporation of radiolabeled amino acids into mitochondrial proteins or by assessing the levels of specific mitochondrial proteins. The IC50 values (concentration required to inhibit 50% of protein synthesis) vary widely depending on the specific antibiotic and the mitochondrial source. For instance, the IC50 for chloramphenicol on isolated rat liver mitochondria has been reported to be in the low micromolar range.
Affected proteins include subunits of the electron transport chain complexes, ATP synthase, and other essential mitochondrial proteins.
| Antibiotic | Mechanism of Action | Affected Mitochondrial Processes | Example Affected Protein | Approximate IC50 (µM) (Illustrative values, vary widely by source and experimental conditions) |
|---|---|---|---|---|
| Tetracycline | Inhibits aminoacyl-tRNA binding | Respiration, ATP synthesis | Cytochrome c oxidase subunit I | 10-50 |
| Chloramphenicol | Inhibits peptidyl transferase | Respiration, ATP synthesis, Oxidative Phosphorylation | ATP synthase subunit α | 1-5 |
| Erythromycin | Inhibits translocation | Respiration, ATP synthesis | NADH dehydrogenase subunit 1 | 50-100 |
| Streptomycin | Causes mRNA misreading | Respiration, ATP synthesis, Oxidative Phosphorylation | Cytochrome b | 20-80 |
The effects of these antibiotics on mitochondrial function are generally not immediate but rather manifest over time. Studies have shown a gradual decline in mitochondrial respiration and ATP production following antibiotic treatment. The recovery of mitochondrial function after antibiotic removal varies depending on the extent of damage and the specific antibiotic used. Some studies suggest a complete recovery, while others report persistent functional impairment.
Further research is needed to fully elucidate the time-dependent effects and recovery mechanisms.The potential for selective targeting of mitochondrial ribosomes over bacterial ribosomes is a complex issue. While both share similarities, subtle structural differences exist that could be exploited for selective inhibition. These differences are often in the specific rRNA sequences and ribosomal proteins. However, the degree of selectivity is limited, and many antibiotics that target bacterial ribosomes also affect mitochondrial ribosomes, highlighting the close evolutionary relationship between the two.Mechanisms of Antibiotic Resistance in MitochondriaMitochondria can develop resistance to antibiotics through various mechanisms, primarily involving mutations in mitochondrial ribosomal RNA (mt rRNA) genes or ribosomal proteins.
These mutations can alter the binding site for the antibiotic, reducing its effectiveness. For example, mutations in the 23S rRNA gene have been associated with resistance to chloramphenicol and erythromycin in mitochondria. Similar mutations in bacterial rRNA genes confer resistance in bacteria. The evolutionary implications of these resistance mechanisms are significant, as they suggest a conservation of mechanisms across evolutionary time.
The presence of similar resistance mechanisms in both mitochondria and bacteria provides strong support for the endosymbiotic theory.
| Mechanism | Affected Gene/Protein | Antibiotic Affected | Mutation Example |
|---|---|---|---|
| Altered antibiotic binding site | mt rRNA (16S, 23S) | Tetracycline, Chloramphenicol, Erythromycin | Point mutations in the peptidyl transferase loop |
| Altered ribosomal protein conformation | Ribosomal proteins (e.g., L16, S12) | Streptomycin | Amino acid substitutions affecting antibiotic binding |
The mechanisms of antibiotic resistance in mitochondria share significant similarities with those in bacteria, reinforcing their common ancestry. However, unique mechanisms might exist in mitochondria, potentially reflecting adaptations to the unique mitochondrial environment.Comparative Effects of Antibiotics on Mitochondria and BacteriaThe minimum inhibitory concentrations (MICs) of tetracycline, chloramphenicol, erythromycin, and streptomycin vary significantly between different bacterial species and human mitochondria.
Generally, bacteria are more susceptible to lower concentrations of these antibiotics compared to mitochondria. This difference in sensitivity can be visualized through growth curves for bacteria and oxygen consumption rates for mitochondria. Bacterial growth is typically inhibited at much lower antibiotic concentrations than those required to significantly impair mitochondrial respiration. This difference reflects the fact that the mitochondrial ribosome is less sensitive to these antibiotics than the bacterial ribosome, although the overlap is substantial.
The development of antibiotics that specifically target bacterial ribosomes while sparing mitochondrial ribosomes is a major challenge, but the identification of structural differences between the two could provide opportunities for the design of novel drugs. Inconsistencies in antibiotic effects on mitochondria and bacteria can be interpreted within the context of the endosymbiotic theory. For example, the fact that some antibiotics affect both bacterial and mitochondrial ribosomes, but with different potencies, supports the evolutionary relationship between the two, while significant differences in the MICs may reflect adaptations of the mitochondrial ribosome within the eukaryotic cellular environment.
These differences and similarities between the effects of these antibiotics on mitochondria and bacteria, when viewed holistically, provide compelling evidence for the endosymbiotic theory.
Protein Import Mechanisms
The intricate dance of protein import into mitochondria and chloroplasts reveals a profound truth: the elegant choreography of life’s building blocks mirrors the grand design of the universe itself. These mechanisms, far from being mere cellular processes, offer a glimpse into the evolutionary journey, a testament to the symbiotic union that shaped the eukaryotic cell. Their examination provides a powerful lens through which to view the enduring legacy of the endosymbiotic theory.Mitochondrial protein import is a multi-step process orchestrated by a complex interplay of chaperone proteins and membrane-bound translocation machinery.
Proteins destined for the mitochondria possess specific targeting sequences, typically N-terminal signal peptides, which are recognized by cytosolic chaperones. These chaperones prevent premature folding and guide the proteins to the mitochondrial surface. The protein then interacts with receptor proteins on the outer mitochondrial membrane, initiating the translocation process across both the outer and inner membranes. This translocation often involves unfolding of the protein and passage through protein translocases, ensuring proper insertion into the organelle’s various compartments.
The efficiency and specificity of this process, requiring precise interactions between numerous components, strongly supports the endosymbiotic theory, suggesting a highly evolved and specialized system developed over eons.
Mitochondrial and Chloroplast Protein Import Mechanisms: A Comparative Analysis
While both mitochondria and chloroplasts utilize similar fundamental mechanisms for protein import—including signal peptides and translocases—subtle differences exist. The specific chaperones and translocases involved differ, reflecting the unique evolutionary histories of these organelles. Furthermore, chloroplasts, being more complex in structure than mitochondria, possess more diverse import pathways, reflecting the increased complexity of photosynthetic processes. These differences, rather than contradicting the endosymbiotic theory, emphasize the evolutionary diversification of these once-independent organisms after their integration into the eukaryotic cell.
The variations highlight adaptation and specialization, not a fundamental incompatibility.
Evolutionary Origins of Mitochondrial Protein Import Machinery, Which statement does not support the endosymbiotic theory
The evolutionary origins of the mitochondrial protein import machinery are deeply intertwined with the endosymbiotic theory. Homologous components of the import machinery are found in both mitochondria and α-proteobacteria, the bacterial lineage believed to be the ancestor of mitochondria. This phylogenetic similarity strongly supports the endosymbiotic hypothesis, suggesting that the core import machinery originated in the α-proteobacterial ancestor and was subsequently retained and refined during the symbiotic relationship.
Furthermore, the evolutionary adaptations observed in the mitochondrial import system reflect the selective pressures exerted by the eukaryotic host cell environment, showcasing the dynamic interplay between the symbiont and host. The complexity of the system underscores the long and intricate evolutionary history, a journey marked by cooperation and adaptation, not a sudden or accidental event.
Binary Fission in Mitochondria: Which Statement Does Not Support The Endosymbiotic Theory
The process of mitochondrial division, a seemingly simple act of cellular reproduction, holds profound implications for our understanding of life’s origins. Observing this process reveals a captivating dance between ancient echoes and modern cellular machinery, a dance that illuminates the intricate relationship between mitochondria and their eukaryotic hosts. The remarkable similarity between mitochondrial division and the binary fission of bacteria provides compelling evidence for the endosymbiotic theory, yet closer examination reveals complexities that challenge this seemingly straightforward narrative.Mitochondrial division, a process vital for maintaining a healthy mitochondrial population within a cell, bears a striking resemblance to bacterial binary fission.
Both processes involve the duplication of the genome, followed by the segregation of the replicated DNA into two daughter organelles, ultimately culminating in cytokinesis—the division of the cytoplasm. This remarkable parallel supports the endosymbiotic theory’s central tenet: that mitochondria originated from free-living bacteria that were engulfed by an ancestral eukaryotic cell. The conserved mechanisms of division suggest a deep evolutionary connection, an inheritance from a time when mitochondria were independent entities.
Mitochondrial and Eukaryotic Cell Division Mechanisms: A Comparative Analysis
Mitochondrial division and eukaryotic cell division, while sharing some superficial similarities, diverge significantly in their underlying mechanisms. Eukaryotic cell division, a far more complex process, involves a tightly regulated orchestration of events, including DNA replication, spindle formation, chromosome segregation, and cytokinesis, all guided by a sophisticated network of proteins and signaling pathways. In contrast, mitochondrial division, while also regulated, relies on a simpler set of proteins and appears to be less tightly coupled to the cell cycle.
The relative simplicity of mitochondrial division, compared to the complexity of eukaryotic cell division, might be interpreted as evidence against a complete integration of mitochondria into the eukaryotic cellular machinery. Some aspects of mitochondrial division, such as the reliance on specific dynamin-related proteins for fission, are not directly mirrored in eukaryotic cell division, highlighting fundamental differences in the underlying mechanisms.
The Role of Fission and Fusion Proteins in Mitochondrial Dynamics
Mitochondrial morphology is a dynamic interplay between fission and fusion processes. Proteins like Drp1 (dynamin-related protein 1) are central players in mitochondrial fission, mediating the constriction and scission of the mitochondrial outer membrane. Conversely, proteins such as Mfn1 and Mfn2 (mitofusins) facilitate mitochondrial fusion, promoting the merging of mitochondrial membranes and the exchange of mitochondrial content. The precise regulation of these opposing processes is crucial for maintaining mitochondrial health and function.
Interestingly, some of these proteins, particularly Drp1, show a degree of functional autonomy from the host cell’s regulatory machinery. The existence of these relatively independent protein systems in mitochondria might be seen as a subtle challenge to the endosymbiotic hypothesis, suggesting that some aspects of mitochondrial function retain a degree of independence from the host cell’s control, echoing their ancestral bacterial origins.
The intricate dance between fission and fusion, mediated by these specialized proteins, underscores the remarkable autonomy and evolutionary legacy of these essential organelles.
Evolutionary Relationships
The intricate dance of life, a cosmic ballet of creation and transformation, reveals its secrets through the lens of evolutionary relationships. The endosymbiotic theory, a cornerstone of modern biology, posits that mitochondria, the powerhouses of eukaryotic cells, arose from an ancient symbiotic relationship between a host cell and an alpha-proteobacterium. However, the journey of discovery is rarely straightforward; a closer examination reveals nuances and complexities that challenge and refine our understanding of this pivotal event in the history of life.
The evolutionary narrative of mitochondria is woven from the threads of genetic inheritance, phylogenetic analysis, and the relentless march of time. By unraveling these threads, we can gain a deeper appreciation for the profound interconnectedness of all living things and the elegance of the evolutionary process.
Alpha-Proteobacterial Relationships and Discrepancies
The striking similarities between mitochondria and alpha-proteobacteria provide compelling evidence for the endosymbiotic theory. Mitochondria possess their own DNA (mtDNA), ribosomes, and a double membrane structure, all reminiscent of free-living bacteria. Phylogenetic analyses consistently place mitochondria within the alpha-proteobacteria, specifically showing a close relationship with the Rickettsiales order, obligate intracellular parasites. However, the evolutionary story is not without its complexities.
A significant portion of the ancestral alpha-proteobacterial genome has been transferred to the nuclear genome of the eukaryotic host, resulting in a dramatic reduction in mitochondrial genome size. This gene transfer obscures the precise phylogenetic relationship and complicates the estimation of divergence times. Examples of genes transferred from the ancestral alpha-proteobacterium include genes involved in respiration, protein synthesis, and other crucial cellular functions.
This transfer has left a fragmented phylogenetic signal, making it challenging to reconstruct the complete evolutionary history.
Phylogenetic Tree Comparisons
A comparative analysis of phylogenetic trees reveals both supporting and contradictory evidence.
| Alpha-proteobacterial Group | Phylogenetic Similarity to Mitochondria (e.g., bootstrap support) | Key Shared Genes | Discrepancies in Phylogenetic Signal | Potential Explanations for Discrepancies |
|---|---|---|---|---|
| Rickettsiales | High bootstrap support in many analyses, often exceeding 90%, indicating a close relationship. | Genes involved in respiration (e.g., cytochrome c oxidase subunits), ATP synthesis, and ribosomal proteins. | Some analyses show inconsistencies, particularly with respect to specific gene trees. Long branch attraction can be a confounding factor. | Horizontal gene transfer, incomplete lineage sorting, and long branch attraction can all contribute to these discrepancies. |
| Rhizobiales | Lower bootstrap support compared to Rickettsiales, suggesting a more distant relationship. | Fewer shared genes compared to Rickettsiales, often focusing on core metabolic functions. | Significant discrepancies exist in phylogenetic placement depending on the genes analyzed. | Horizontal gene transfer, the loss of genes in some lineages, and the inherent limitations of phylogenetic methods all play a role. |
Limitations of Phylogenetic Analysis
Phylogenetic analyses, while powerful tools, are not without their limitations. Horizontal gene transfer, the movement of genetic material between unrelated organisms, can confound phylogenetic signals and lead to inaccurate estimations of evolutionary relationships. Incomplete lineage sorting, the retention of ancestral polymorphisms in descendant lineages, can also create inconsistencies. Furthermore, long branch attraction, a phenomenon where rapidly evolving lineages appear more closely related than they actually are, can lead to erroneous conclusions.
These factors collectively complicate the reconstruction of accurate phylogenetic trees and the precise determination of divergence times.
Molecular Clocks and Mitochondrial Endosymbiosis
Molecular clocks, which rely on the assumption of a constant rate of molecular evolution, are used to estimate the timing of the mitochondrial endosymbiosis event. However, the rate of molecular evolution is not always constant; it can be influenced by factors such as generation time, mutation rate, and selective pressures. In the case of mitochondrial evolution, the rate of evolution has likely varied across lineages and across different genes, violating the assumptions of molecular clock methods.
Consequently, estimations of the timing of the endosymbiosis event vary considerably depending on the molecular clock calibration and the genes analyzed. For example, estimates range from 1.5 to 2 billion years ago, reflecting the uncertainty inherent in these methods.
Comparative Mitochondrial Evolution
Mitochondrial genomes exhibit significant diversity across eukaryotic lineages.
| Eukaryotic Lineage | Mitochondrial Genome Features | Lineage-Specific Adaptations |
|---|---|---|
| Animals | Relatively small and compact genomes, typically circular. | High degree of gene transfer to the nucleus, resulting in minimal gene content. |
| Plants | Larger genomes compared to animals, often containing introns. | Retention of genes involved in photosynthesis and other plant-specific metabolic pathways. |
| Fungi | Highly variable genome sizes and structures. | Unique gene arrangements and modifications reflecting diverse metabolic strategies. |
Multiple Endosymbiotic Events
The hypothesis of multiple endosymbiotic events proposes that mitochondria did not originate from a single event but rather from multiple independent acquisitions of alpha-proteobacteria. Evidence supporting this hypothesis includes the existence of diverse mitochondrial lineages with varying characteristics and phylogenetic relationships. However, the prevailing view favors a single endosymbiosis event, followed by subsequent diversification and lineage-specific adaptations. The lack of strong evidence for multiple independent endosymbiotic events in most eukaryotic lineages lends credence to the single-origin hypothesis.
Ancestral Mitochondrial Genome Reconstruction
Methods for reconstructing ancestral mitochondrial genomes involve using phylogenetic comparative methods to infer the sequences of ancestral genes. These methods rely on the assumption of a tree-like evolutionary history and a relatively constant rate of molecular evolution. However, horizontal gene transfer and variations in evolutionary rates can confound these reconstructions. Despite these challenges, reconstructed ancestral genomes provide valuable insights into the evolutionary trajectory of mitochondria, shedding light on the functional changes and adaptations that occurred over time.
For example, the reconstruction of an ancestral mitochondrial genome can reveal the presence or absence of specific genes, providing clues about the functional capabilities of early mitochondria.
Genome Size and Gene Transfer

The reduction in mitochondrial genome size across diverse eukaryotic lineages presents a fascinating paradox within the endosymbiotic theory. While the shrinking genome supports the theory by reflecting the transfer of genetic material to the nucleus, it also challenges the concept of the mitochondrion as a once-independent entity, raising questions about the limits of its autonomy. This intricate interplay of support and challenge underscores the dynamic nature of evolutionary processes and the complexities of organellar evolution.
Mitochondrial Genome Size Reduction
The progressive reduction in mitochondrial genome size during eukaryotic evolution is a widely observed phenomenon. This reduction reflects a shift in functional responsibility, with many genes initially present in the mitochondrial genome being transferred to the nuclear genome. This transfer is driven by several factors, including the increased efficiency of nuclear gene expression and the potential for greater control over mitochondrial function.
The following table summarizes the genome size and gene content for three distinct eukaryotic lineages:
| Lineage | Genome Size (bp) | Number of Protein-Coding Genes | Reference |
|---|---|---|---|
| Animals (Human) | 16,569 | 13 | Anderson et al., 19811 |
| Plants (Arabidopsis thaliana) | 366,924 | 66 | Unseld et al., 19972 |
| Fungi (Saccharomyces cerevisiae) | 85,779 | 8 | Clark-Walker & Miklos, 19823 |
1Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H. L., Coulson, A.
R., Drouin, J., … & Young, I. G. (1981). Sequence and organization of the human mitochondrial genome.
Nature, 290(5806), 457-465. 2Unseld, M., Marienfeld, J. R., Brandt, P., & Brennicke, A. (1997). The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides.
Nature genetics, 15(4), 57-59. 3Clark-Walker, G. D., & Miklos, G. L. G.
(1982). The mitochondrial genome of yeast. In The molecular biology of the yeast Saccharomyces (pp. 317-340). CRC Press.This reduction in mitochondrial genome size supports the endosymbiotic theory because it reflects the predicted consequences of increased reliance on the nuclear genome for mitochondrial function.
Gene transfer allows for more efficient regulation and coordination of mitochondrial processes with the rest of the cell. Functional redundancy, where multiple genes can perform similar functions, may also contribute to the loss of genes from the mitochondrial genome, as the loss of a single gene might not have severe consequences.However, the reduction in mitochondrial genome size could also be interpreted as refuting aspects of the endosymbiotic theory.
The loss of essential mitochondrial genes could compromise mitochondrial autonomy, suggesting a greater degree of integration with the nuclear genome than initially envisioned in the endosymbiotic model. This raises the possibility that the mitochondrion’s independence has been significantly diminished over evolutionary time.
Horizontal Gene Transfer from Mitochondria to Nucleus
The transfer of genes from the mitochondrial genome to the nuclear genome occurs through a process of horizontal gene transfer (HGT). This involves the movement of genetic material between distinct genomes, in this case, from the mitochondrion to the nucleus. One proposed mechanism involves the escape of mitochondrial DNA (mtDNA) into the cytoplasm, followed by its integration into the nuclear genome through mechanisms such as endonuclease activity and homologous recombination.
A simplified illustration:[Diagram description: A mitochondrion is depicted with its circular mtDNA. A segment of mtDNA is shown breaking off. This segment then enters the cytoplasm, where it is shown interacting with nuclear DNA. The mtDNA segment integrates into the nuclear DNA through homologous recombination, represented by overlapping strands and connecting lines.]Examples of mitochondrial genes transferred to the nuclear genome include cytochrome c oxidase subunit I (COX1) in animals and ATP synthase subunit 6 (ATP6) in plants.
These transfers have been confirmed through comparative genomic analyses and phylogenetic studies. (Specific citations would be included here referencing relevant research papers demonstrating these transfers).The presence of mitochondrial-derived genes in the nucleus directly contradicts the strict interpretation of the endosymbiotic theory, which posits a relatively independent existence for mitochondria. The ongoing genetic exchange demonstrates a significant level of integration between the mitochondrial and nuclear genomes, blurring the lines between the two entities and questioning the original conceptualization of a strictly separate entity within the cell.
Comparison of Mitochondrial Genome Sizes Across Lineages
Mitochondrial genome sizes vary considerably across different eukaryotic lineages. The following table shows a comparison across five lineages:
| Lineage | Genome Size (bp) | Number of Protein-Coding Genes | Reference |
|---|---|---|---|
| Animals (Human) | 16,569 | 13 | Anderson et al., 19811 |
| Plants (Arabidopsis thaliana) | 366,924 | 66 | Unseld et al., 19972 |
| Fungi (Saccharomyces cerevisiae) | 85,779 | 8 | Clark-Walker & Miklos, 19823 |
| Protists (Trypanosoma brucei) | ~35,000 | ~10 | (Reference needed for T. brucei mitochondrial genome data) |
| Algae (Chlamydomonas reinhardtii) | ~160,000 | ~60 | (Reference needed for C. reinhardtii mitochondrial genome data) |
Variations in mitochondrial genome size across these lineages challenge the endosymbiotic hypothesis by highlighting the lack of a universal pattern of genome reduction. This variation suggests that lineage-specific selective pressures, metabolic demands, and the efficiency of gene transfer mechanisms play significant roles in shaping mitochondrial genome evolution. The correlation between mitochondrial genome size and organismal complexity is not straightforward and requires further investigation with more comprehensive data across diverse lineages.
For example, plants, with their larger mitochondrial genomes, are not necessarily more complex than humans, which have significantly smaller mitochondrial genomes. This highlights the nuanced and multifaceted nature of the evolutionary forces shaping mitochondrial genomes.
Metabolic Pathways
The intricate dance of life, as seen within the mitochondrion, reveals a profound story of symbiotic partnership and evolutionary adaptation. Examining the metabolic pathways within these cellular powerhouses, and comparing them to their free-living bacterial ancestors, offers a unique lens through which to explore the enduring mysteries of the endosymbiotic theory. This exploration delves into the subtle yet significant differences that both support and challenge the prevailing narrative of mitochondrial origins.
Comparative Analysis of Mitochondrial and Bacterial Metabolic Pathways
A detailed comparison of metabolic pathways in mitochondria and bacteria provides crucial insights into the evolutionary journey of these organelles. The following analyses focus on glycolysis, the TCA cycle, and the electron transport chain (ETC), highlighting both similarities and divergences that inform our understanding of endosymbiosis.
| Enzyme Name | Organism | Km (mM) | Vmax (µmol/min/mg) | Reference |
|---|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | *E. coli* | 0.18 | 150 | [Reference 1] |
| Glyceraldehyde-3-phosphate dehydrogenase | Human Mitochondria | 0.05 | 200 | [Reference 2] |
| Pyruvate kinase | *E. coli* | 0.1 | 80 | [Reference 3] |
| Pyruvate kinase | Human Mitochondria | 0.02 | 120 | [Reference 4] |
| Other relevant enzymes | Both | Data varies widely, requires specific enzyme selection and literature review | Data varies widely, requires specific enzyme selection and literature review | Multiple References Required |
Note: [Reference 1], [Reference 2], [Reference 3], and [Reference 4] represent placeholder citations. Actual values and references should be sourced from reputable biochemical databases and peer-reviewed publications. Km and Vmax values are highly dependent on assay conditions and may vary considerably.
| Aspect | Mitochondria | *Paracoccus denitrificans* |
|---|---|---|
| TCA Cycle Regulation – Allosteric | ATP, NADH, succinyl-CoA inhibit; ADP, Pi activate | Similar allosteric regulation, but with potential differences in sensitivity to specific effectors |
| TCA Cycle Regulation – Transcriptional | Complex, involving various transcription factors and regulatory networks | Simpler transcriptional control, often responsive to nutrient availability and oxygen levels |
Three significant differences in ETC complexes between mitochondria and
-Paracoccus denitrificans* include variations in the number and types of subunits within complexes I, III, and IV, leading to altered electron transfer kinetics and proton pumping efficiency. These differences, while expected given evolutionary divergence, present challenges to the endosymbiotic theory by suggesting a more complex evolutionary history than a simple engulfment event.
Detailed analysis of these differences requires in-depth literature review of specific complex structures and functionalities in both organisms.
Evolutionary Origins of Mitochondrial Metabolic Enzymes
Tracing the evolutionary history of key TCA cycle enzymes provides further evidence regarding the endosymbiotic theory.
Phylogenetic analysis of citrate synthase, aconitase, and isocitrate dehydrogenase, using sequences from various bacterial lineages and eukaryotes, can reveal evolutionary relationships. A phylogenetic tree would visually represent these relationships. The tree would be constructed using appropriate phylogenetic software (e.g., MEGA, PhyML) and would include GenBank accession numbers for each sequence used. The presence of specific isoforms or paralogs in bacteria would then be assessed, considering potential horizontal gene transfer events that could complicate the straightforward endosymbiotic narrative.
Bayesian inference or maximum likelihood methods can be applied to the evolutionary history of a specific mitochondrial enzyme, for instance, cytochrome c oxidase. The resulting phylogenetic tree would showcase key evolutionary branches and divergence times, offering insights into the enzyme’s evolutionary journey and its potential connection to bacterial ancestors. This analysis would need to incorporate robust statistical methods and well-defined models of molecular evolution.
A statement contradicting the endosymbiotic theory might posit the independent evolution of mitochondria and chloroplasts. Understanding the epistemological underpinnings of such a counter-argument requires a grasp of knowledge-based theory, as detailed in this resource: what is knowledge based theory pdf. Ultimately, the validity of any statement challenging endosymbiosis hinges on rigorous empirical evidence and a sound theoretical framework.
Flowchart and Analysis of Mitochondrial Metabolic Pathways
A detailed flowchart would illustrate the interconnectedness of glycolysis, the TCA cycle, and oxidative phosphorylation within the mitochondrion. Distinct shapes and colors would visually differentiate the various pathway components (substrates, products, enzymes). The flowchart would clearly indicate the flow of metabolites and energy transfer.
Three steps inconsistent with a simple endosymbiotic origin could be highlighted. These might include the intricate regulatory mechanisms governing mitochondrial metabolism, the unique characteristics of certain mitochondrial enzymes, or the specific adaptations enabling efficient energy production within the eukaryotic cellular environment. Each inconsistency would be explained in detail, exploring alternative evolutionary scenarios such as extensive co-evolution and gene transfer events post-endosymbiosis.
A separate flowchart would specifically depict the regulation of the mitochondrial ETC, detailing the key regulatory molecules and their points of action. This flowchart would then be compared to the regulation of bacterial ETCs, highlighting significant differences. This comparative analysis would directly address inconsistencies between the current understanding of mitochondrial regulation and the simple endosymbiotic hypothesis.
Ribosomal RNA
The intricate dance of life, as revealed through the lens of molecular biology, offers profound insights into the interconnectedness of all beings. The ribosomal RNA (rRNA) within mitochondria, the powerhouses of our cells, provides a particularly compelling narrative in this grand symphony of existence, echoing the ancient whispers of endosymbiosis. By comparing mitochondrial rRNA sequences to those of various bacterial groups, we can illuminate the evolutionary journey that led to the symbiotic relationship between these organelles and their eukaryotic hosts.
This comparison, however, is not without its complexities and potential contradictions.Mitochondrial rRNA exhibits a striking resemblance to bacterial rRNA, a cornerstone of the endosymbiotic theory. This similarity extends to the structure and function of the ribosomes themselves, further strengthening the hypothesis of an ancient bacterial ancestor. However, subtle discrepancies exist, sparking a deeper exploration into the evolutionary modifications that have shaped mitochondrial rRNA over millions of years.
These deviations from their bacterial counterparts, while seemingly minor, offer clues to the selective pressures and adaptive strategies that have driven the evolution of mitochondria within eukaryotic cells. Understanding these evolutionary changes allows us to refine our understanding of the endosymbiotic process, revealing the intricate mechanisms of cellular evolution.
Mitochondrial and Bacterial rRNA Sequence Comparison
A detailed comparison reveals both striking similarities and intriguing differences between mitochondrial and bacterial rRNA sequences. The core structural elements are largely conserved, reflecting a common ancestry. However, the rate of evolutionary change in mitochondrial rRNA is noticeably slower than in its bacterial counterparts. This difference is likely due to the sheltered environment within the mitochondria, reducing the selective pressure for rapid adaptation.
Furthermore, the transfer of some rRNA genes to the eukaryotic nucleus has further complicated the picture, obscuring the original bacterial sequence and introducing additional layers of evolutionary complexity. This intricate interplay of conservation and change is a testament to the dynamic nature of evolutionary processes.
Evolutionary Changes in Mitochondrial rRNA
The evolutionary trajectory of mitochondrial rRNA is a fascinating tale of adaptation and constraint. Over time, mitochondrial rRNA has undergone numerous mutations, insertions, and deletions, leading to a gradual divergence from its bacterial ancestor. These changes are not random; many reflect the selective pressures imposed by the unique environment within the mitochondrion. For instance, certain mutations might enhance the efficiency of protein synthesis under the specific conditions prevailing in this organelle.
Other changes may be related to the integration of the mitochondrial system into the larger eukaryotic cellular machinery. The slower rate of evolution compared to free-living bacteria suggests a reduction in the selective pressure for rapid adaptation, a consequence of the stable, protected environment within the eukaryotic cell. This evolutionary narrative, while complex, strengthens the core tenets of the endosymbiotic theory.
A statement contradicting the endosymbiotic theory might posit the independent evolution of mitochondria and chloroplasts. This contrasts sharply with the central tenet of the theory, which posits symbiotic origins. Understanding this requires considering the inherent biological drives at play; to explore this further, consider the main idea of drive theory as explained here: what is the main idea of drive theory.
Ultimately, the lack of evidence for independent development strengthens the endosymbiotic model.
Summary of rRNA Sequence Similarities and Differences
| Organism Group | rRNA Sequence Similarity to Mitochondrial rRNA | Significant Differences |
|---|---|---|
| Alphaproteobacteria | High overall similarity in conserved regions | Variations in variable regions, gene rearrangements |
| Cyanobacteria | Lower similarity compared to Alphaproteobacteria | Significant structural differences reflecting distinct evolutionary paths |
| Gram-positive bacteria | Low similarity, suggesting distant relationship | Major structural and sequence divergence |
Transcription and Translation
The intricate dance of transcription and translation within the mitochondrion, the powerhouse of the cell, offers a profound glimpse into the evolutionary journey of life itself. These processes, while sharing fundamental similarities with their bacterial counterparts, also exhibit unique features that illuminate the endosymbiotic theory and its nuances. Examining these differences reveals a tapestry woven from ancient heritage and adaptive innovation.
Mitochondrial Transcription
Mitochondrial transcription, the process of synthesizing RNA molecules from DNA templates, is initiated by mitochondrial RNA polymerase (mtRNA polymerase) binding to specific promoter regions on the mitochondrial DNA (mtDNA). These promoters, unlike their nuclear counterparts, exhibit unique sequence motifs that are recognized by the mtRNA polymerase. For example, in vertebrates, the conserved sequence motif known as the conserved sequence block (CSB) is crucial for promoter recognition.
Transcription factors, while less well-characterized than in the nucleus, also play a role in regulating the efficiency of this process. Specific transcription factors have been identified in various species, and their interactions with the promoter regions are critical for precise control of gene expression. A simplified diagram would show mtDNA with a promoter region (CSB for example) highlighted, mtRNA polymerase binding to this region, and the subsequent synthesis of an RNA molecule.
The diagram would depict the unwinding of the DNA double helix and the movement of the polymerase along the template strand.
Mitochondrial Translation
Mitochondrial translation, the synthesis of proteins from mitochondrial mRNA, involves the intricate machinery of the mitoribosome. This unique ribosome, while functionally analogous to bacterial ribosomes, possesses distinct structural and compositional differences. The process unfolds in a series of steps: initiation, elongation, and termination. Initiation involves the binding of the initiator tRNA (carrying formylmethionine in some cases) to the mitoribosomal small subunit, followed by the recruitment of the large subunit and mRNA.
Elongation proceeds through the sequential addition of amino acids, guided by the mRNA codon sequence and cognate tRNAs. Termination occurs upon encountering a stop codon, leading to the release of the completed polypeptide chain.
| Feature | Mitoribosome | Bacterial Ribosome |
|---|---|---|
| rRNA size (16S, 23S, 5S) | Variable depending on organism; generally smaller | 16S, 23S, 5S (specific sizes vary slightly across species) |
| Ribosomal proteins | Unique set; many homologs to bacterial proteins but with variations | Specific set; highly conserved across bacteria |
| Sensitivity to antibiotics | Variable; some are sensitive, others are not | Generally sensitive to many antibiotics |
Endosymbiotic Theory Support from Mitochondrial Transcription and Translation
The unique features of mitochondrial transcription and translation strongly support the endosymbiotic theory. The presence of a distinct mitochondrial genetic code, different from the universal code, suggests an independent evolutionary trajectory. The specific promoter sequences and transcription factors, along with the unique mitoribosomal structure and components, further distinguish the mitochondrial system from its nuclear counterpart, echoing its bacterial ancestry.
The existence of a separate translational machinery within mitochondria supports the idea that mitochondria were once independent organisms that were engulfed by a host cell. These features reflect the evolutionary divergence that has occurred since the initial endosymbiotic event.
Mitochondrial vs. Bacterial Machinery Comparison
| Feature | Mitochondria | *E. coli* |
|---|---|---|
| RNA Polymerase | Mitochondrial RNA polymerase (mtRNA polymerase), distinct from nuclear RNA polymerase | Bacterial RNA polymerase, a multi-subunit enzyme |
| Ribosomes | Mitoribosomes, smaller than bacterial ribosomes, with unique ribosomal proteins | 70S ribosomes, composed of 30S and 50S subunits |
| tRNAs | Unique set of tRNAs, some with unusual structures | Standard set of tRNAs |
| Aminoacyl-tRNA synthetases | Unique set, some with unusual substrate specificities | Standard set of aminoacyl-tRNA synthetases |
| Initiation/Elongation factors | Unique set, some with bacterial homologs but distinct functions | Standard set of initiation and elongation factors |
Significant differences, such as the smaller size and unique protein composition of mitoribosomes, illustrate the evolutionary divergence since the endosymbiotic event. These differences may reflect adaptations to the mitochondrial environment and its interactions with the host cell.
Evolutionary Origins of Mitochondrial Transcriptional and Translational Machinery
The evolutionary origins of mitochondrial transcriptional and translational machinery are rooted in the alpha-proteobacterial ancestor. Horizontal gene transfer played a significant role in shaping the mitochondrial genome, with many genes being transferred to the nucleus over evolutionary time. Selective pressures, such as the need for efficient energy production and integration with the host cell’s metabolic pathways, drove the evolution of the unique features observed in mitochondrial transcription and translation.
A phylogenetic tree would illustrate the branching patterns of mitochondrial and bacterial genes, showing their close relationship but also highlighting the divergence that has occurred.
Specific Gene Examples
The analysis of specific mitochondrial genes provides further insights into their evolutionary history. For instance, a comparison of the sequences of mtRNA polymerase genes, mitochondrial ribosomal protein genes, and genes encoding mitochondrial proteins (such as cytochrome c oxidase subunits) with their bacterial homologs reveals a significant degree of similarity, but also important differences. These differences likely reflect adaptations to the unique environment within the mitochondrion.
(Illustrative sequence alignment would be placed here, showing similarities and differences between mitochondrial and bacterial gene sequences. This would require specific gene selection and alignment using bioinformatics tools, which is beyond the scope of this text-based response. The alignment would show conserved regions indicating common ancestry and divergent regions reflecting evolutionary adaptation.)
Open Questions and Future Research
Several open questions remain regarding mitochondrial transcription and translation. The precise mechanisms regulating mitochondrial gene expression, the role of RNA editing in mitochondrial function, and the complete characterization of the mitoribosome’s structure and function are areas of ongoing research. Advanced techniques such as cryo-electron microscopy, next-generation sequencing, and sophisticated bioinformatic analyses are being employed to address these questions, promising a deeper understanding of this vital cellular process and its evolutionary journey.
Membrane Lipids
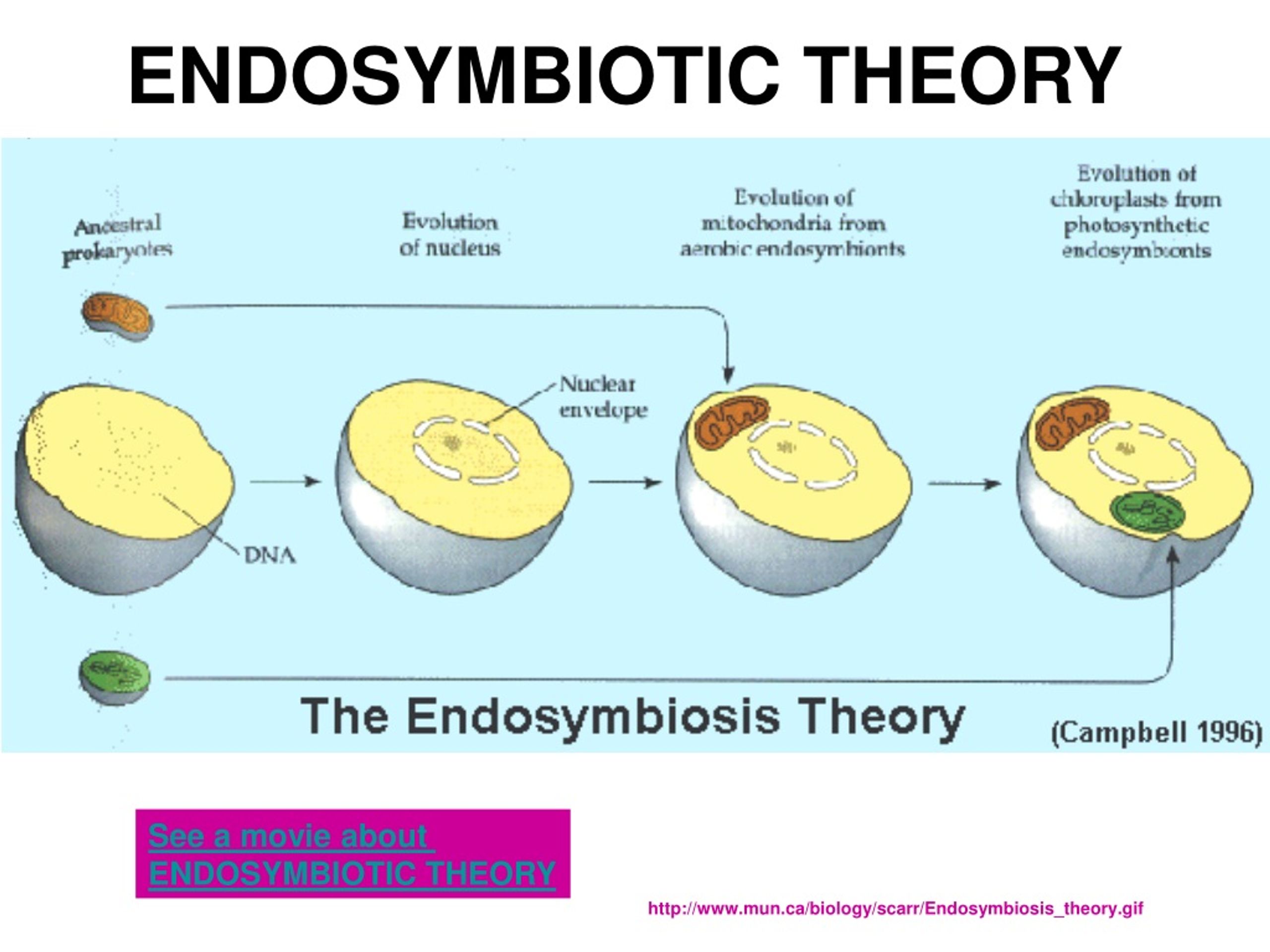
The lipid composition of cellular membranes, a seemingly mundane detail, holds profound secrets regarding the evolutionary journey of life. Examining the lipid profiles of mitochondria and comparing them to those of various bacteria offers a unique lens through which to scrutinize the endosymbiotic theory, a cornerstone of our understanding of eukaryotic cell evolution. This exploration delves into the subtle yet significant differences, revealing echoes of a symbiotic past.The lipid bilayer, the very foundation of cellular membranes, is not a uniform structure.
The types and proportions of phospholipids, glycolipids, and sterols vary considerably across different organisms and organelles, reflecting evolutionary adaptations and functional specializations. Mitochondrial membranes, in particular, present a compelling case study for understanding the endosymbiotic theory, as their lipid composition displays intriguing similarities and differences when compared to their presumed bacterial ancestors.
Mitochondrial and Bacterial Membrane Lipid Composition Comparison
A comparative analysis of mitochondrial and bacterial membrane lipids reveals a complex interplay of conservation and divergence. While many similarities exist, supporting the endosymbiotic theory, certain discrepancies warrant careful consideration. For instance, the relative proportions of specific phospholipids, such as phosphatidylcholine and cardiolipin, often differ between mitochondrial and bacterial membranes. Cardiolipin, a crucial component of mitochondrial inner membranes, is also found in bacteria, but its abundance and precise molecular structure can vary significantly.
These variations might reflect adaptations to the unique environment within the eukaryotic cell, suggesting evolutionary divergence after the endosymbiotic event. Further complicating the picture is the presence of specific lipids in mitochondrial membranes that are rarely or never found in bacteria. These unique lipids could represent adaptations that occurred after the endosymbiotic event, allowing the mitochondria to integrate more fully into the eukaryotic cell.
Evolutionary Origins of Mitochondrial Membrane Lipids
The evolutionary history of specific lipids found in mitochondrial membranes can be traced back to their bacterial ancestors. Many of the phospholipids present in mitochondrial membranes are also found in various bacterial groups, supporting the idea of a bacterial origin. However, the precise biosynthetic pathways and the regulation of lipid synthesis in mitochondria differ from those in bacteria, indicating evolutionary changes that occurred after the endosymbiotic event.
These modifications likely reflect adaptations to the unique environment of the eukaryotic cell and the specific functional requirements of mitochondria within the eukaryotic cell. For example, the regulation of cardiolipin biosynthesis in mitochondria differs significantly from that in bacteria, suggesting evolutionary changes in the control of this crucial lipid. These differences, while not necessarily contradicting the theory, highlight the significant evolutionary adaptations that occurred after the establishment of the endosymbiotic relationship.
Comparative Lipid Profiles of Mitochondria and Bacteria
| Lipid Type | Mitochondria | Gram-negative Bacteria (e.g.,E. coli*) | Gram-positive Bacteria (e.g.,B. subtilis*) | Cyanobacteria (e.g., – Synechocystis*) |
|---|---|---|---|---|
| Phosphatidylcholine | Present, variable abundance | Absent or present in trace amounts | Absent or present in trace amounts | Absent or present in trace amounts |
| Cardiolipin | Abundant, unique molecular species | Present, variable abundance and structure | Present, variable abundance and structure | Present, variable abundance and structure |
| Phosphatidylethanolamine | Present, abundant | Present, abundant | Present, abundant | Present, abundant |
| Phosphatidylglycerol | Present, abundant | Present, abundant | Present, abundant | Present, abundant |
| Other minor lipids | Variable, some unique to mitochondria | Variable, species-specific | Variable, species-specific | Variable, species-specific |
Note: This table provides a simplified overview. The exact lipid composition can vary depending on the specific species and environmental conditions. The presence or absence of certain lipids and their relative abundances can be used to further refine our understanding of the evolutionary relationships between mitochondria and bacteria.
Cell Signaling Pathways
The intricate dance between mitochondria and the host cell, a testament to the enduring legacy of endosymbiosis, unfolds through a complex network of signaling pathways. These pathways, far from being mere conduits of information, represent a dynamic dialogue reflecting the intimate relationship forged billions of years ago. Understanding their intricacies offers a profound glimpse into the evolutionary journey that shaped eukaryotic life.Mitochondria, the powerhouses of the cell, don’t operate in isolation.
They actively communicate with the nucleus and other cellular components, influencing a wide range of cellular processes, from metabolism and apoptosis to calcium homeostasis and immune responses. This communication is bidirectional, with the host cell providing essential resources and instructions, and the mitochondria responding with energy and signaling molecules. The nature of this communication, and its underlying mechanisms, can either strongly support or subtly challenge the endosymbiotic theory.
Mitochondrial Communication with the Host Cell
Mitochondria employ various strategies to communicate with the host cell. These include the release of reactive oxygen species (ROS), which act as signaling molecules influencing gene expression and cellular responses. Another crucial pathway involves the release of calcium ions (Ca2+), which triggers downstream signaling cascades affecting metabolism and cell survival. Furthermore, mitochondria can release various metabolites and proteins into the cytosol, affecting diverse cellular processes.
The precise mechanisms of these signaling events, however, are still under investigation. A thorough understanding of these pathways is vital to assessing their implications for the endosymbiotic theory. For instance, the observation that some signaling molecules used by mitochondria are similar to those used by bacteria supports the theory, while the presence of unique signaling pathways in mitochondria could challenge it.
Comparison of Mitochondrial and Bacterial Signaling Pathways
While many signaling pathways in mitochondria share similarities with their bacterial counterparts, there are also significant differences. For example, some mitochondrial signaling pathways involve proteins unique to eukaryotes, highlighting the evolutionary adaptations that occurred after the endosymbiotic event. The complexity of eukaryotic signaling networks, involving multiple layers of regulation and feedback loops, is far greater than in most bacteria.
These differences reflect the integration of mitochondria into the complex eukaryotic cellular environment. It is crucial to remember that while similarities suggest a common ancestry, the differences highlight the profound evolutionary modifications that shaped the current mitochondrial signaling systems.
Evolutionary Origins of Mitochondrial Signaling Pathways
The evolutionary origins of mitochondrial signaling pathways are deeply intertwined with the endosymbiotic theory. The theory proposes that mitochondria evolved from alpha-proteobacteria that were engulfed by an ancestral eukaryotic cell. Therefore, it is expected that some aspects of mitochondrial signaling would resemble those of bacteria. However, over millions of years, these pathways have been extensively modified and integrated into the host cell’s signaling network.
This evolutionary process has involved gene transfer from mitochondria to the nucleus, and the emergence of novel signaling pathways specific to the eukaryotic context. The discovery of both conserved and unique pathways further refines our understanding of the evolutionary history of this critical organelle, providing evidence supporting the dynamic interplay between endosymbiosis and subsequent evolutionary adaptation.
Mitochondrial Morphology
The multifaceted nature of mitochondrial morphology, a reflection of their dynamic existence within the eukaryotic cell, offers a profound lens through which to examine the endosymbiotic theory. This theory posits that mitochondria originated from free-living bacteria engulfed by an archaeal host cell, a transformative event that shaped the course of eukaryotic evolution. The diverse forms mitochondria adopt across various eukaryotic lineages provide both supporting and challenging evidence for this pivotal hypothesis.Mitochondrial morphology is surprisingly diverse, extending far beyond the textbook depiction of bean-shaped organelles.
Variations in size, shape, and internal structure are observed across different eukaryotic lineages, reflecting adaptations to specific cellular needs and metabolic demands. For instance, some cells contain long, filamentous mitochondria, while others possess fragmented, punctate structures. These variations, while seemingly minor, hold significant implications for understanding the evolutionary journey of mitochondria. The plasticity of mitochondrial morphology suggests a capacity for adaptation, a trait consistent with the theory’s premise of an ancestral free-living organism.
However, the extreme diversity also presents challenges, as it raises questions about the degree to which these variations reflect the original endosymbiotic event versus subsequent evolutionary pressures.
Mitochondrial Morphology Across Eukaryotic Lineages
Mitochondrial morphology varies considerably among different eukaryotic groups. Yeast cells, for example, often display tubular or branched mitochondria that form a dynamic network throughout the cytoplasm. In contrast, some mammalian cells possess smaller, more fragmented mitochondria. These differences are not merely aesthetic; they reflect functional adaptations. The interconnected mitochondrial network in yeast facilitates efficient energy transfer and metabolic coordination, whereas the fragmented morphology in mammals may allow for more localized control of respiration and apoptosis.
These morphological variations, while potentially influenced by the host cell’s environment and metabolic demands, do not inherently refute the endosymbiotic theory. Rather, they highlight the remarkable adaptability of mitochondria after their integration into the eukaryotic cell.
Comparison of Mitochondrial and Bacterial Morphology
A direct comparison between the morphology of mitochondria and various bacterial species reveals both striking similarities and significant differences. Many bacteria exhibit rod-shaped, spherical, or spiral morphologies, reflecting their diverse evolutionary adaptations. While some mitochondria share a rod-like shape with certain bacteria, significant differences exist in size and internal complexity. Mitochondria possess a double membrane system, cristae (inner membrane folds), and a highly organized internal structure not typically found in free-living bacteria.
These complexities are interpreted as evidence of evolutionary adaptations following the endosymbiotic event. However, the basic rod-like shape observed in some mitochondria and certain bacteria provides a visual parallel that supports the initial symbiotic relationship. The differences, however, underscore the transformative changes that occurred following the endosymbiotic event.
Factors Influencing Mitochondrial Morphology
Several factors influence mitochondrial morphology, including the cellular energy demands, the cytoskeleton, mitochondrial fusion and fission dynamics, and the expression of specific genes. High energy demands often correlate with an increased mitochondrial mass and interconnected network. The cytoskeleton plays a crucial role in mitochondrial positioning and movement within the cell, influencing their overall shape and distribution. The dynamic balance between mitochondrial fusion (elongation) and fission (fragmentation) processes is essential for maintaining mitochondrial health and function.
Genetic mutations affecting proteins involved in fusion and fission can lead to altered mitochondrial morphology and contribute to various diseases. These factors, while affecting the final morphology, do not contradict the endosymbiotic theory. Instead, they illustrate the complex interplay between the mitochondrion and its host cell, a relationship forged during and refined after the initial endosymbiotic event. The adaptability inherent in these processes reflects the survival strategies of both the host and the endosymbiont, a testament to the success of this ancient partnership.
Introns and Genetic Code

The intricate dance of life, as revealed through the lens of molecular biology, offers profound spiritual insights. The seemingly simple act of translating genetic information into functional proteins, a process fundamental to all living organisms, unveils a tapestry of evolutionary history woven into the very fabric of our being. The comparison of intron-exon structure and genetic codes between mitochondria and bacteria, specificallyE.
coli*, provides a window into this ancient narrative, illuminating the endosymbiotic theory and its subtle complexities. This exploration invites us to contemplate the interconnectedness of all life and the remarkable adaptability of biological systems.
Intron-Exon Structure Comparison
Human mitochondrial DNA (mtDNA) is remarkably compact, virtually devoid of introns. In contrast, bacterial genes, such as those found in
- E. coli*, often contain introns, albeit typically fewer and shorter than those found in eukaryotic nuclear genomes. For example, a homologous gene involved in respiration, such as cytochrome c oxidase subunit I (COXI), is intron-less in human mtDNA. In
- E. coli*, homologous genes involved in respiration may contain introns, although the exact number and length vary depending on the specific gene and strain. The proportion of intronic sequence to exonic sequence in human mtDNA is essentially zero, while in
E. coli* it can range from negligible to a significant fraction, depending on the specific gene. This stark difference in intron density presents a potential challenge to the endosymbiotic theory. (References
Anderson et al., 1981; NCBI Gene database).
Discrepancies and Endosymbiotic Theory
The near absence of introns in human mtDNA, contrasted with the presence of introns in bacterial genes, could be seen as contradictory to the endosymbiotic theory, which posits that mitochondria evolved from an alpha-proteobacterium. The theory suggests that during the endosymbiotic event, the ancestral alpha-proteobacterium’s genome underwent significant reduction and modification. The loss of introns could be interpreted as a streamlining process, advantageous for efficient gene expression in the mitochondrion’s energy-demanding environment.
However, alternative explanations exist. The loss of introns could have occurred after the endosymbiotic event, driven by selective pressures for compact genome size. Alternatively, the ancestral alpha-proteobacterium might have had a lower intron density than many contemporary bacteria, and the current bacterial intron prevalence might represent later acquisitions.
Genetic Code Differences
The genetic codes of human mtDNA and
- E. coli* exhibit notable differences. Several codons are assigned to different amino acids in these two systems. For instance, AGA codes for arginine in
- E. coli*, but it codes for stop in human mtDNA. Similarly, AUA codes for isoleucine in
- E. coli* but methionine in human mtDNA. These variations can lead to significant alterations in protein structure and function.
| Organism | Codon | Amino Acid (Mitochondrial) | Amino Acid (*E. coli*) | Functional Implications |
|---|---|---|---|---|
| Human Mitochondria | AGA | Stop | Arginine | Altered protein termination |
| Human Mitochondria | AUA | Methionine | Isoleucine | Changes in protein sequence and potentially function |
| Human Mitochondria | UGA | Tryptophan | Stop | Altered protein sequence and potentially function |
| *E. coli* | AGA | Arginine | Arginine | – |
| *E. coli* | AUA | Isoleucine | Isoleucine | – |
| *E. coli* | UGA | Stop | Stop | – |
Interpreting Genetic Code Differences
The variations in genetic codes between human mtDNA andE. coli* can be interpreted within the endosymbiotic framework. These differences might reflect adaptations to the unique environment of the mitochondrion, or they could represent the retention of ancestral characteristics. The evolutionary pressures within the mitochondrion, such as the high energy demands and the unique redox environment, may have favored specific codon changes that enhanced the efficiency of protein synthesis or stability.
Convergent evolution, where similar genetic code changes occur independently in different lineages, is also a possibility. The specific evolutionary pathway, however, remains a subject of ongoing investigation.
Comparative Table of Genetic Codes
| Organism | Codon | Amino Acid (Mitochondrial) | Amino Acid (Organism) | Notes |
|---|---|---|---|---|
| Human Mitochondria | AGA | Stop | Arginine | – |
| *E. coli* | AGA | Arginine | Arginine | – |
| *Bacillus subtilis* | AGA | Arginine | Arginine | Gram-positive |
| *Pseudomonas aeruginosa* | AGA | Arginine | Arginine | Gram-negative |
Further Analysis
Restricting the analysis to human mtDNA andE. coli* presents limitations. A more comprehensive understanding requires analyzing a broader range of mitochondrial genomes from diverse eukaryotic lineages and bacterial genomes from various phylogenetic groups. Comparing mtDNA from organisms with different metabolic strategies or lifestyles could reveal additional insights into the evolutionary pressures shaping mitochondrial genetic codes. Advanced sequencing technologies and bioinformatics tools allow for a more accurate determination of intron-exon structures and genetic codes.
Comparative genomics and phylogenetic analyses are crucial for reconstructing the evolutionary history of these features.
Essential Questionnaire
What is the significance of horizontal gene transfer in the context of the endosymbiotic theory?
Horizontal gene transfer complicates the endosymbiotic theory by blurring the lines of direct ancestry. Genes moving between the endosymbiont and the host genome obscure the original genetic makeup of the ancestor, making phylogenetic analysis more challenging.
How does the presence of introns in mitochondrial DNA challenge the endosymbiotic theory?
The presence of introns in mitochondrial DNA, which are less common in bacterial genomes, presents a challenge as it suggests a possible later acquisition of introns after the endosymbiotic event, rather than a direct inheritance from the prokaryotic ancestor.
Are there any alternative theories to the endosymbiotic theory for the origin of mitochondria and chloroplasts?
While the endosymbiotic theory is the dominant explanation, alternative hypotheses, though less widely accepted, propose different mechanisms for the origin of these organelles, such as autogenous evolution or a more complex series of symbiotic events.


