Does translation in protein synthesis support the theory of evolution – Does translation in protein synthesis support the theory of evolution? Totally! Think of it like this: our DNA is the blueprint for life, but it’s the proteins that actually
-do* stuff – build tissues, fight infections, even make you crave that extra nasi goreng. Translation is the crucial step where the DNA code gets converted into these proteins.
Tiny errors in this process – mutations – can create variations in proteins, some beneficial, some not so much. These variations are the raw material for natural selection, the driving force behind evolution. It’s a super cool, intricate process that’s been shaping life on Earth for billions of years.
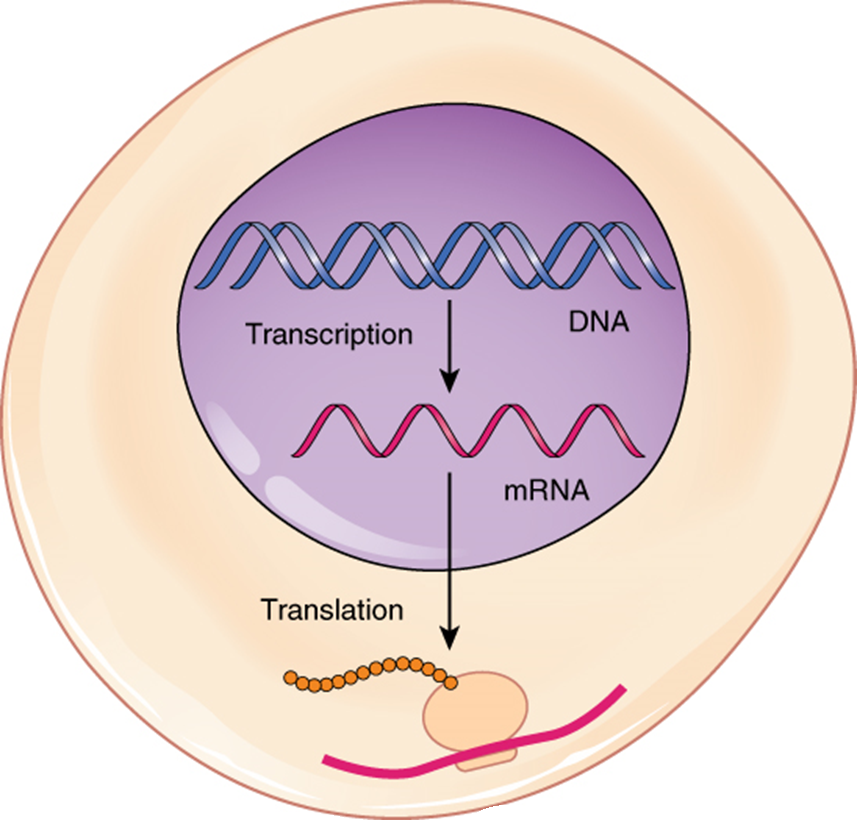
The central dogma of molecular biology – DNA makes RNA makes protein – is fundamental to understanding how this works. Transcription copies the DNA code into messenger RNA (mRNA), which then acts as a template for translation. Transfer RNA (tRNA) molecules bring the correct amino acids to the ribosome, the protein-making machinery, based on the mRNA code. Each three-letter codon on the mRNA specifies a particular amino acid.
The sequence of codons determines the sequence of amino acids in the resulting protein, directly impacting its structure and function. Mutations, alterations in the DNA sequence, can change the mRNA and ultimately the protein. A single amino acid change can have huge consequences, from creating a slightly different protein with a new function to completely disrupting the protein’s structure and rendering it useless.
This process of mutation and selection over vast periods has driven the incredible biodiversity we see today.
Introduction to Protein Synthesis and the Genetic Code
Protein synthesis, the process of building proteins from genetic information, is fundamental to life and provides a powerful lens through which to view evolution. The central dogma of molecular biology, DNA → RNA → Protein, underpins this process and explains how changes in DNA can lead to variations in proteins, driving evolutionary change.
The Central Dogma and its Relevance to Evolution
The central dogma describes the flow of genetic information within a biological system. DNA, the hereditary material, serves as a template for RNA synthesis (transcription), and RNA, specifically messenger RNA (mRNA), directs protein synthesis (translation). Mutations, alterations in the DNA sequence, are the raw material of evolution. These changes can be as small as a single nucleotide alteration (point mutation) or as large as chromosomal rearrangements.
Point mutations, for instance, can significantly impact protein structure and function. A missense mutation alters a single codon, resulting in a different amino acid in the protein. This can lead to changes in protein folding, stability, and ultimately, its function. A nonsense mutation introduces a premature stop codon, leading to a truncated and often non-functional protein.
Protein synthesis, specifically the translation process, provides compelling evidence for evolution by showcasing the conserved mechanisms across diverse species. Understanding how this fundamental biological process operates helps clarify the distinction between scientific theory and hypothesis; to learn more, consult this resource on which of the following distinguishes a theory from a hypothesis. The universality of the genetic code strongly supports the evolutionary theory of common ancestry, highlighting the shared evolutionary history reflected in the translation machinery itself.
A silent mutation, however, changes a codon but codes for the same amino acid, resulting in no change in the protein’s sequence. The effect on evolutionary fitness depends on the nature and location of the mutation, and the environmental context. A mutation that confers an advantage in a specific environment will be favored by natural selection, leading to its increased frequency within a population.
| Mutation Type | Effect on Codon | Effect on Amino Acid | Effect on Protein | Example |
|---|---|---|---|---|
| Missense | Changes a single nucleotide | Changes the amino acid | May alter protein structure and function | Sickle cell anemia (Glu to Val in hemoglobin) |
| Nonsense | Changes a codon to a stop codon | Premature termination of translation | Truncated, often non-functional protein | Many genetic diseases |
| Silent | Changes a codon, but codes for the same amino acid | No change in amino acid | No change in protein sequence or function | Many variations in DNA sequences |
Transcription and Translation
Transcription is the process of synthesizing an RNA molecule from a DNA template. It involves three stages: initiation, elongation, and termination. Initiation begins with RNA polymerase binding to a promoter region on the DNA. Transcription factors help regulate this binding. Elongation involves the sequential addition of ribonucleotides to the growing RNA strand.
Termination signals the end of transcription. A simplified diagram would show DNA unwinding, RNA polymerase moving along the template strand, and the newly synthesized mRNA molecule detaching.Translation is the process of synthesizing a polypeptide chain from an mRNA template. The mRNA molecule, carrying the genetic code in the form of codons (three-nucleotide sequences), interacts with ribosomes. Ribosomes, composed of large and small subunits, facilitate the binding of transfer RNA (tRNA) molecules.
Each tRNA molecule carries a specific amino acid and an anticodon, complementary to an mRNA codon. Aminoacyl-tRNA synthetases attach the correct amino acid to its corresponding tRNA. The ribosome moves along the mRNA, matching codons to anticodons, and catalyzes the formation of peptide bonds between adjacent amino acids. Release factors recognize stop codons, signaling the termination of translation and the release of the completed polypeptide chain.
A step-by-step illustration would show the ribosome binding to mRNA, tRNA molecules entering the A and P sites, peptide bond formation, translocation, and release of the polypeptide.Post-translational modifications, such as glycosylation (addition of sugar molecules) and phosphorylation (addition of phosphate groups), further modify the protein’s structure and function, affecting its activity, stability, and localization within the cell.
The Genetic Code and its Universality
The genetic code is a set of rules specifying the correspondence between codons in mRNA and amino acids in a polypeptide chain. Its universality means that the same codons code for the same amino acids in most organisms. However, exceptions exist. For example, some mitochondria and certain bacteria use slightly different codons. This degeneracy, where multiple codons specify the same amino acid, is a feature of the genetic code, adding robustness to the system.
A table showcasing the degeneracy would list amino acids and the corresponding codons.
| Amino Acid | Codons |
|---|---|
| Leucine | UUA, UUG, CUU, CUC, CUA, CUG |
| Serine | UCU, UCC, UCA, UCG, AGU, AGC |
Protein Synthesis: A Summary
Protein synthesis is a remarkably intricate and tightly regulated process that bridges the gap between genotype and phenotype. It begins with the transcription of DNA into mRNA, a process orchestrated by RNA polymerase and transcription factors. The mRNA molecule, carrying the genetic blueprint, then moves to the ribosome, the protein synthesis machinery. Here, tRNA molecules, each carrying a specific amino acid, decode the mRNA codons, bringing the correct amino acids to the ribosome in the correct sequence.
The ribosome, a complex molecular machine, catalyzes the formation of peptide bonds between adjacent amino acids, assembling the polypeptide chain. Once synthesized, the polypeptide chain undergoes folding and often post-translational modifications, which are essential for achieving its final functional three-dimensional structure. The accuracy of this process is critical; errors in any step—from DNA replication to post-translational modifications—can lead to non-functional or misfolded proteins, potentially causing disease.
For example, a single nucleotide change in the DNA sequence can lead to a missense mutation, altering the amino acid sequence and potentially the function of the resulting protein. This change can be detrimental, beneficial, or have no effect depending on the location and type of mutation, as well as the environment. The universality of the genetic code underscores the fundamental unity of life, while the exceptions highlight the evolutionary adaptations of specific organisms.
The entire process reflects a remarkable interplay between the genetic information encoded in DNA and the complex molecular machinery of the cell.
Translation Fidelity and Evolutionary Change
/protein_synthesis-57c4c3795f9b5855e5038563.jpg)
The accuracy of protein synthesis, or translation fidelity, is crucial for cellular function and organismal survival. Errors in translation can lead to the production of non-functional proteins, impacting a wide range of cellular processes and potentially causing disease. Furthermore, the mechanisms ensuring translation accuracy have evolved over time, reflecting selective pressures for efficient and precise protein synthesis. Understanding these mechanisms and their evolution provides valuable insights into the relationship between genotype and phenotype and the broader process of evolution.
Aminoacyl-tRNA Synthetase Editing and Ribosomal Quality Control
Aminoacyl-tRNA synthetases (aaRS) are essential enzymes that attach the correct amino acid to its corresponding tRNA molecule. This process, called aminoacylation, is remarkably accurate, with error rates typically less than 1 in 10,000. This high fidelity is achieved through multiple mechanisms, including a two-step reaction with an editing site that removes incorrectly attached amino acids. The editing site hydrolyzes the incorrect aminoacyl-adenylate before it’s transferred to the tRNA.
This proofreading step significantly reduces the overall error rate. Ribosomes also possess quality control mechanisms. They can detect and sometimes correct errors such as frameshift mutations or the incorporation of incorrect amino acids. These mechanisms, often involving GTP hydrolysis, enhance the overall accuracy of translation.
mRNA Secondary Structure and Translational Fidelity
The secondary structure of mRNA, formed by base pairing within the molecule, can influence ribosome progression and potentially lead to errors in translation. Hairpin loops or other complex structures can cause ribosomes to pause or stall, increasing the chance of misincorporation or premature termination. Cells have evolved mechanisms to mitigate these effects. For instance, chaperone proteins can bind to mRNA and help to maintain its structure or assist in resolving ribosome stalls.
Similarly, RNA helicases can unwind mRNA secondary structures, facilitating efficient ribosome movement. An example is the effect of mRNA secondary structures near the start codon, which can influence the efficiency of translation initiation.
Comparison of Translation Fidelity Across Cellular Compartments
Translation fidelity varies across different cellular compartments. Cytoplasmic translation generally exhibits higher fidelity than translation in mitochondria or chloroplasts. This difference is likely due to several factors, including differences in the accuracy of the aaRS enzymes and ribosomal components in these organelles. Mitochondria and chloroplasts, having evolved from endosymbiotic bacteria, retain some features of prokaryotic translation machinery, which may be less precise than the highly evolved eukaryotic cytoplasmic machinery.
The selection pressures for high fidelity are also likely different in different cellular compartments, with the cytoplasm requiring higher accuracy for essential cellular processes.
Prokaryotic versus Eukaryotic Translation Machinery
Prokaryotic and eukaryotic ribosomes differ significantly in their structure and function. Prokaryotic ribosomes (70S) are smaller and simpler than eukaryotic ribosomes (80S), with differences in their rRNA composition and associated proteins. These differences extend to the initiation, elongation, and termination factors involved in translation. For example, prokaryotic translation initiation involves the Shine-Dalgarno sequence and initiation factors IF1, IF2, and IF3, while eukaryotic initiation involves the Kozak sequence and eukaryotic initiation factors (eIFs) like eIF2, eIF4, and eIF5.
These differences in translation machinery have evolutionary implications, impacting translation speed, fidelity, and regulation. For instance, the faster translation rate in prokaryotes may be linked to a lower fidelity compared to eukaryotes.
Ribosomal RNA (rRNA) in Translation and Evolutionary Conservation
rRNA plays crucial structural and functional roles in ribosome assembly and function. The 16S rRNA in prokaryotes and the 18S rRNA in eukaryotes are key components of the small ribosomal subunit, responsible for mRNA binding and decoding. The 23S rRNA in prokaryotes and the 28S rRNA in eukaryotes are part of the large ribosomal subunit, involved in peptidyl transfer and translocation.
rRNA sequences and structures are highly conserved across different species, reflecting the fundamental importance of ribosomes in protein synthesis. Conserved regions often correspond to functionally important sites within the ribosome. These conserved rRNA sequences are invaluable for phylogenetic analyses, allowing researchers to infer evolutionary relationships between different organisms based on ribosome evolution. Mutations in rRNA can have severe consequences, affecting translation fidelity and ribosome function.
For example, certain rRNA mutations are associated with human diseases, including ribosomopathies.
Consequences of Translation Errors
Mistranslation leads to the production of non-functional proteins or proteins with altered function. This can trigger cellular stress responses, protein aggregation, and potentially cell death. The accumulation of misfolded proteins can overwhelm the cell’s protein quality control systems, leading to various cellular dysfunctions. A link exists between translation errors and various diseases, including cancer and neurodegenerative disorders. The cell employs various mechanisms to cope with misfolded or aggregated proteins, including chaperones, proteasomes, and autophagy.
Stalled ribosomes are also detected and addressed by cellular mechanisms, including ribosome rescue factors that help to remove stalled ribosomes and prevent further damage. These mechanisms represent potential therapeutic targets for diseases associated with translation errors.
Amino Acid Selection and Protein Structure
The selection of specific amino acids during translation is a crucial step determining the final protein’s structure and function. This process isn’t random; it’s dictated by the genetic code, the mRNA sequence, and the intricate interplay of various cellular components. The precise sequence of amino acids, in turn, dictates how the protein folds into its unique three-dimensional shape, which is essential for its biological activity.The sequence of amino acids directly influences protein structure through several levels of organization.
The primary structure is simply the linear sequence of amino acids dictated by the mRNA. This sequence then folds into secondary structures, like alpha-helices and beta-sheets, stabilized by hydrogen bonds between the amino acid backbones. Further folding, driven by interactions between amino acid side chains (R-groups), leads to the tertiary structure, a three-dimensional arrangement defining the protein’s overall shape.
Finally, some proteins have quaternary structure, formed by the association of multiple polypeptide chains. The properties of each amino acid—size, charge, polarity, and hydrophobicity—play a critical role in determining which structures form and how stable they are. A change in even a single amino acid can dramatically alter the protein’s structure and, consequently, its function. For example, a single amino acid substitution in the hemoglobin protein leads to sickle cell anemia.
Amino Acid Properties and Their Impact on Protein Folding
The properties of amino acids significantly influence protein folding. Hydrophobic amino acids tend to cluster in the protein’s interior, away from the aqueous environment, while hydrophilic amino acids are often found on the surface. Charged amino acids can form ionic bonds, contributing to protein stability. The size and shape of amino acids also influence how tightly they pack together, affecting the protein’s overall three-dimensional structure.
| Amino Acid | Property | Impact on Folding | Example |
|---|---|---|---|
| Glycine (Gly) | Small, nonpolar | Increases flexibility in the polypeptide chain | Found in regions of proteins requiring flexibility. |
| Proline (Pro) | Cyclic structure, rigid | Introduces kinks and bends in the polypeptide chain | Often found at the turns of alpha-helices or beta-sheets. |
| Cysteine (Cys) | Contains a thiol group (-SH) | Forms disulfide bonds, stabilizing protein structure | Crucial for the stability of many extracellular proteins. |
| Lysine (Lys) | Positively charged | Participates in ionic interactions and hydrogen bonding | Often found on the protein surface, interacting with negatively charged molecules. |
Protein Folding and Evolutionary Relationships
Protein folding is a crucial process in cellular biology, directly impacting protein function and ultimately, an organism’s survival. The intricate three-dimensional structure of a protein is dictated by its amino acid sequence and is essential for its biological activity. Understanding protein folding mechanisms and how they evolve across species provides valuable insights into the molecular basis of life and the evolutionary processes that have shaped it.
Protein Folding Process and its Importance
Protein folding is the process by which a polypeptide chain attains its unique three-dimensional structure. This structure is not random; it’s precisely determined by the amino acid sequence, a consequence of the genetic code. The process involves several levels of structural organization:
- Primary structure: This refers to the linear sequence of amino acids linked by peptide bonds. The primary structure dictates all subsequent levels of organization.
- Secondary structure: Local folding patterns stabilized by hydrogen bonds between the backbone atoms. Common secondary structures include alpha-helices and beta-sheets.
- Tertiary structure: The overall three-dimensional arrangement of a polypeptide chain, stabilized by various interactions including hydrogen bonds, disulfide bridges (covalent bonds between cysteine residues), hydrophobic interactions (clustering of nonpolar amino acids), and ionic bonds.
- Quaternary structure: The arrangement of multiple polypeptide chains (subunits) to form a functional protein complex. Interactions between subunits are similar to those stabilizing tertiary structure.
The amino acid sequence, determined by the gene, acts as a blueprint for the protein’s final three-dimensional structure. Hydrophobic amino acids tend to cluster in the protein’s interior, away from the aqueous environment, while hydrophilic amino acids are usually found on the surface. This hydrophobic effect is a major driving force in protein folding. Disulfide bridges, formed between cysteine residues, contribute to the stability of the folded structure.Protein misfolding, where the protein fails to attain its correct three-dimensional structure, can have severe consequences.
Misfolded proteins can be non-functional, or they may aggregate, forming insoluble clumps that can damage cells and tissues. Several diseases are caused by protein misfolding, including:
- Alzheimer’s disease: Characterized by the aggregation of amyloid-beta plaques in the brain.
- Parkinson’s disease: Involves the aggregation of alpha-synuclein proteins in Lewy bodies.
- Cystic fibrosis: Results from misfolding of the cystic fibrosis transmembrane conductance regulator (CFTR) protein.
[Diagram: A simple diagram could show a linear polypeptide chain gradually folding into a compact globular structure. Labels should indicate alpha-helices, beta-sheets, disulfide bridges, hydrophobic core, and hydrophilic surface. The diagram should illustrate the transition from primary to tertiary structure.]
The Role of Chaperone Proteins
Chaperone proteins are essential molecular machines that assist in the proper folding of other proteins. They prevent aggregation and promote the formation of the native, functional conformation. Several classes of chaperones exist, including:
- Hsp70 (Heat shock protein 70): These are ATP-dependent chaperones that bind to unfolded or misfolded proteins, preventing aggregation.
- Hsp60 (also known as chaperonins): These form large barrel-shaped complexes that provide a protected environment for protein folding.
- Small heat shock proteins (sHsps): These bind to unfolded proteins, preventing aggregation, and can transfer their bound substrates to other chaperones.
Hsp70 chaperones function through a cycle of ATP binding and hydrolysis. ATP binding causes a conformational change that allows Hsp70 to bind to unfolded proteins. ATP hydrolysis then causes another conformational change, increasing the chaperone’s affinity for the substrate and promoting folding. Upon ATP rebinding, Hsp70 releases the folded protein.Chaperones prevent protein aggregation by binding to exposed hydrophobic patches on unfolded proteins, shielding them from interacting with other proteins.
This prevents the formation of large, insoluble aggregates.
| Chaperone Type | Mechanism | Target Proteins |
|---|---|---|
| Hsp70 | ATP-dependent binding and release; prevents aggregation | Wide range of unfolded or misfolded proteins |
| Hsp60 (Chaperonins) | Provides a protected environment for folding; ATP-dependent | Proteins requiring assistance with complex folding |
| sHsps | Binds unfolded proteins, preventing aggregation; transfers substrates to other chaperones | Variety of unfolded or stress-denatured proteins |
Comparative Analysis of Protein Folding Pathways
Comparing protein folding pathways across different species reveals both conserved elements and species-specific adaptations. For example, comparing the folding pathways of
- E. coli* (a bacterium) and
- Homo sapiens* (a mammal) reveals that while the basic principles of protein folding are conserved, there are differences in the specific chaperones utilized and the efficiency of folding. Many homologous proteins, proteins with shared ancestry, exhibit similar folding pathways and structural motifs.
Conserved amino acid residues and structural motifs are crucial for protein folding. These conserved elements often reside in regions critical for the protein’s stability and function. Mutations affecting these conserved regions can lead to misfolding and loss of function. For example, mutations in the conserved hydrophobic core of a protein can destabilize the folded structure, making it more susceptible to aggregation.The evolutionary implications of conserved folding pathways are significant.
The conservation of folding mechanisms across diverse species suggests that these mechanisms are highly efficient and have been selectively maintained throughout evolution. Variations in folding pathways likely reflect adaptations to different environmental conditions or cellular processes.
| Feature | E. coli | Homo sapiens | Example Protein |
|---|---|---|---|
| Major Chaperones | DnaK (Hsp70 homolog), GroEL/ES (Hsp60 homolog) | Hsp70, Hsp60, other chaperones | Ribosomal proteins (highly conserved across species) |
| Folding Pathways | Generally faster, less complex | More complex, involving a wider array of chaperones | Cytochrome c (conserved structure, slight variations in folding pathways) |
| Conserved Elements | Hydrophobic core, specific secondary structures | Similar hydrophobic core, similar secondary structures | Actin (conserved structure and folding mechanism) |
| Impact of Mutations | Can lead to misfolding and reduced growth | Can lead to disease or dysfunction | Beta-globin (mutations causing sickle cell anemia) |
Mutations and Their Impact on Translation
Mutations are alterations in the DNA sequence that can significantly impact the process of translation, ultimately affecting the amino acid sequence and function of the resulting protein. These changes form the raw material for evolution, providing the variation upon which natural selection acts. Understanding the types of mutations and their consequences is crucial to grasping the link between molecular changes and evolutionary adaptations.
Types of Mutations Affecting Translation
Point mutations, insertions, and deletions are the primary types of mutations that alter the genetic code and subsequently influence translation. These mutations can occur in various parts of a gene, including the coding sequence, promoter region, or introns, with varying consequences depending on their location and type.
| Mutation Type | DNA Sequence Change | mRNA Codon Change | Amino Acid Change | Potential Functional Consequence |
|---|---|---|---|---|
| Substitution (Transition) | Original: ATC; Mutated: ACC | Original: AUG; Mutated: ACG | Original: Methionine; Mutated: Threonine | May alter protein function depending on the amino acid’s role. |
| Substitution (Transversion) | Original: ATC; Mutated: AGC | Original: AUG; Mutated: UCG | Original: Methionine; Mutated: Serine | May alter protein function depending on the amino acid’s role. |
| Insertion | Original: ATCG; Mutated: ATACG | Original: AUGC; Mutated: AUAGC | Frameshift; complete alteration of downstream amino acid sequence. | Likely significant disruption of protein function; often results in premature stop codon. |
| Deletion | Original: ATCG; Mutated: ATG | Original: AUGC; Mutated: AUG | Frameshift; complete alteration of downstream amino acid sequence. | Likely significant disruption of protein function; often results in premature stop codon. |
Missense mutations result in a change in a single amino acid; nonsense mutations introduce a premature stop codon, truncating the protein; and silent mutations have no effect on the amino acid sequence due to the redundancy of the genetic code. For example, a missense mutation might change a glutamic acid to a valine, potentially altering protein shape and function (as seen in sickle cell anemia).
A nonsense mutation could lead to a non-functional protein, while a silent mutation might have no noticeable impact.Frameshift mutations, caused by insertions or deletions that are not multiples of three nucleotides, shift the reading frame, dramatically altering the amino acid sequence downstream of the mutation.Consider this example: Original DNA sequence: ATC GGA TCC… (Ile Gly Ser…) Insertion of an “A” after the first codon: ATA TCG GAT CC… (Ile Ser Asp…) The entire sequence following the insertion is altered.
A visual representation would show the original codons neatly grouped into triplets, while the mutated sequence shows a shift in the grouping, leading to completely different codons and amino acids after the insertion point.
Impact on Amino Acid Sequences and Protein Function
The impact of a mutation on protein function is highly dependent on the type of mutation, its location within the gene, and the nature of the amino acid change. Mutations in the coding region directly affect the amino acid sequence, while those in promoter regions can alter gene expression levels. Intron mutations may or may not affect the final protein, depending on whether they affect splicing.Conservative amino acid substitutions replace one amino acid with another that has similar chemical properties (e.g., both are hydrophobic), often resulting in less severe functional consequences than non-conservative substitutions, which replace an amino acid with one having very different properties (e.g., a hydrophobic amino acid with a charged one).
For instance, substituting a leucine (nonpolar) with an isoleucine (also nonpolar) is conservative, while substituting it with an aspartic acid (polar, negatively charged) is non-conservative.Mutations can affect protein stability by disrupting the hydrophobic core or altering disulfide bond formation. They can also impact protein folding, leading to misfolded or aggregated proteins. These changes can result in loss of enzymatic activity, altered interactions with other molecules, or even complete loss of function.
Examples of Mutations Leading to Evolutionary Adaptations
1. Lactose Tolerance in Humans
A mutation in theLCT* gene, allowing continued production of lactase into adulthood, conferred a selective advantage in populations that relied on dairy farming. This mutation led to the ability to digest lactose, providing a readily available source of nutrients. (Enattah et al., 2002).
2. Antibiotic Resistance in Bacteria
Mutations in bacterial genes encoding antibiotic target proteins (e.g., ribosomes, enzymes involved in cell wall synthesis) can confer resistance to antibiotics. These mutations often involve amino acid substitutions that alter the binding site of the antibiotic, reducing its effectiveness. (Davies & Davies, 2010).
3. Sickle Cell Anemia and Malaria Resistance
A point mutation in the β-globin gene, leading to the substitution of valine for glutamic acid in hemoglobin, causes sickle cell anemia. However, individuals with one copy of the mutated gene exhibit increased resistance to malaria, providing a selective advantage in malaria-endemic regions. (Allison, 1954).
Horizontal Gene Transfer and Translation
Horizontal gene transfer (HGT), also known as lateral gene transfer, is a significant evolutionary mechanism that plays a crucial role in shaping the genetic diversity of organisms, particularly prokaryotes. Unlike vertical gene transfer, where genetic material is passed from parent to offspring, HGT involves the transfer of genetic material between unrelated organisms. This process has profound implications for protein synthesis, influencing the evolution of both the genetic code and the translational machinery itself.
It introduces new genes encoding novel proteins, modifies existing translation machinery, and ultimately contributes to the adaptation and diversification of life.HGT can introduce new genes encoding proteins with entirely new functions, or genes that modify the existing translation process. This can involve the acquisition of genes for novel ribosomal proteins, tRNAs, aminoacyl-tRNA synthetases, or other components of the translation machinery.
These modifications can lead to altered translation fidelity, efficiency, or even the ability to translate novel codons. For example, the acquisition of antibiotic resistance genes through HGT is a well-documented phenomenon where bacteria gain the ability to synthesize proteins that confer resistance to specific antibiotics. These genes often encode enzymes that modify or destroy the antibiotic, impacting the overall effectiveness of treatment.
Mechanisms of Horizontal Gene Transfer and Their Impact on Protein Synthesis
HGT occurs through several mechanisms, each with the potential to alter protein synthesis. Transformation involves the uptake of free DNA from the environment. Transduction utilizes bacteriophages (viruses that infect bacteria) to transfer genetic material between cells. Conjugation is a direct transfer of DNA between two cells through a physical connection. The acquisition of a new gene via any of these methods could directly impact protein synthesis if the gene encodes a component of the translational apparatus (like a ribosomal protein or a tRNA synthetase) or if it encodes a protein that regulates translation.
For instance, the acquisition of a gene for a new tRNA synthetase could allow the organism to incorporate a non-canonical amino acid into its proteins, expanding its proteome and potentially leading to novel functions. Conversely, the acquisition of a gene encoding a translational repressor could alter the expression levels of many proteins, significantly impacting cellular function.
A Flowchart Illustrating Horizontal Gene Transfer and its Impact on Protein Synthesis
Imagine a flowchart:[Start] –> [Bacterial Cell 1: possesses its own translation machinery] –> [HGT event occurs (Transformation, Transduction, or Conjugation)] –> [Acquisition of new gene (e.g., a novel ribosomal protein gene or a gene for a new aminoacyl-tRNA synthetase)] –> [Integration of new gene into bacterial genome] –> [Transcription and Translation of new gene] –> [Modification of translation machinery or synthesis of new protein] –> [Altered protein synthesis efficiency, fidelity, or expansion of protein repertoire] –> [Potential evolutionary advantage (e.g., antibiotic resistance, adaptation to new environments)] –> [End]This flowchart illustrates a simplified version.
The actual process can be far more complex, involving multiple genes, regulatory interactions, and potential evolutionary consequences. The integration of the new gene might not always be successful, and the effects on protein synthesis can vary greatly depending on the specific gene acquired and the cellular context. Nevertheless, the flowchart clearly demonstrates how HGT can directly influence the evolution of protein synthesis.
Evolution of the Ribosome
The ribosome, a complex molecular machine crucial for protein synthesis, stands as a testament to the power of evolutionary processes. Its intricate structure and conserved yet diverse components across life forms offer compelling evidence for its ancient origins and subsequent diversification. Understanding the ribosome’s evolution provides crucial insights into the history of life on Earth and the mechanisms driving evolutionary change at the molecular level.The ribosome is a ribonucleoprotein particle, meaning it’s composed of both RNA and protein.
Its primary function is to translate the genetic code carried by messenger RNA (mRNA) into the amino acid sequence of a polypeptide chain. This process involves decoding mRNA codons, recruiting transfer RNAs (tRNAs) carrying the appropriate amino acids, and catalyzing peptide bond formation. The ribosome is remarkably conserved across all three domains of life—Bacteria, Archaea, and Eukarya—yet subtle variations exist that reflect evolutionary divergence.
This conservation, alongside the observed differences, provides a rich source of data for phylogenetic analysis and evolutionary inferences.
Ribosomal Structure and Function
Ribosomes are composed of two subunits: a large subunit and a small subunit. The small subunit is primarily responsible for mRNA binding and codon recognition, while the large subunit catalyzes peptide bond formation. Both subunits are comprised of ribosomal RNA (rRNA) molecules, which form the structural core, and numerous ribosomal proteins, which enhance stability and function. The precise number and type of proteins vary across species, but the overall architecture remains remarkably consistent.
For example, bacterial ribosomes (70S) have a 30S small subunit and a 50S large subunit, while eukaryotic ribosomes (80S) have a 40S small subunit and a 60S large subunit. The “S” values refer to Svedberg units, which reflect sedimentation rates during ultracentrifugation and are a measure of size and shape, not additive.
Evolutionary Origins of Ribosomal Components
The evolutionary history of the ribosome is complex and not fully understood. However, the prevailing hypothesis suggests that the ribosome’s RNA components predate its protein components. The RNA world hypothesis proposes that early life relied heavily on RNA for both catalytic and informational functions. Evidence for this includes the catalytic role of rRNA in peptide bond formation (peptidyl transferase activity) and the structural importance of rRNA in forming the ribosome’s core.
Over time, proteins were incorporated, enhancing the ribosome’s efficiency and functionality. The specific evolutionary pathways leading to the modern ribosome are still under investigation, but comparative genomics and structural analyses of ribosomes from various organisms are continuously revealing new details.
Evidence for Evolutionary Relationships Between Ribosomal Subunits
The striking similarities in ribosomal structure and function across all three domains of life strongly support a common ancestral origin. Phylogenetic analyses of rRNA sequences, particularly the highly conserved sequences within the catalytic core, consistently reveal a branching pattern that mirrors the established phylogenetic relationships between organisms. Furthermore, the presence of homologous ribosomal proteins across diverse lineages, albeit with variations in sequence and number, provides further evidence for a common ancestor.
Comparative structural studies have identified conserved structural motifs and domains within both the large and small ribosomal subunits, supporting the notion of shared ancestry and subsequent diversification through evolutionary processes like gene duplication and divergence. For example, specific rRNA secondary structures are remarkably conserved, highlighting the functional importance of these structures and their preservation across evolutionary time. Variations in ribosomal proteins are often associated with adaptations to specific environmental conditions or translational requirements, illustrating the adaptive potential of this crucial cellular machinery.
Translation and Adaptation to Different Environments: Does Translation In Protein Synthesis Support The Theory Of Evolution
Translation, the process of protein synthesis, is not a static process; it’s highly dynamic and adaptable, playing a crucial role in an organism’s ability to survive and thrive in diverse environments. Environmental pressures, such as temperature fluctuations, nutrient limitations, and salinity changes, can significantly impact translation efficiency and accuracy, leading to selective pressures that drive evolutionary changes in the translation machinery itself.
This adaptation ensures the continued production of functional proteins under challenging conditions.
Changes in Translation Efficiency and Environmental Adaptation
Changes in ribosomal speed and accuracy directly impact an organism’s fitness in various environments. Faster translation rates can be advantageous in rapidly changing environments where quick protein production is essential for survival, while higher accuracy is crucial in environments where errors in protein synthesis can be detrimental. For instance, studies have shown a positive correlation between increased translation speed and growth rate in bacteria under nutrient-rich conditions.
Conversely, slower, more accurate translation may be favored in environments with high stress, where the cost of errors is high. A hypothetical graph could depict a parabolic relationship: optimal fitness is achieved at an intermediate translation speed, with both excessively slow and fast rates resulting in reduced fitness. The peak of this parabola would shift depending on the specific environmental stress; under high temperature stress, the peak might shift toward slower, more accurate translation to minimize misfolded proteins.
Quantifying this relationship requires detailed studies correlating specific translation rates (measured, for example, via ribosome profiling) with fitness metrics (e.g., growth rate, survival rate) across a range of stress conditions. Such studies are actively being conducted in various model organisms.
Examples of Specialized Translation Mechanisms
The following table details specific organisms and their evolved translation mechanisms to cope with environmental stress.
| Organism | Environment | Specialized Mechanism | Measurable Improvement | Reference |
|---|---|---|---|---|
| Escherichia coli | High temperature (42°C) | Increased expression of heat shock proteins (HSPs), which assist in protein folding and prevent aggregation | Increased survival rate and faster recovery from heat shock | [Citation needed – A relevant study onE. coli* heat shock response and translation] |
| Psychrobacter arcticus | Low temperature (-10°C) | Modified ribosomal proteins with increased flexibility, allowing for efficient translation at low temperatures | Increased growth rate at low temperatures | [Citation needed – A relevant study on cold-adapted bacteria and ribosomal modifications] |
| Halobacterium salinarum | High salinity (saturated NaCl) | Specialized tRNAs and aminoacyl-tRNA synthetases adapted to high salt concentrations | Maintained protein synthesis efficiency under high salinity | [Citation needed – A relevant study on halophilic archaea and translation adaptation] |
Environmental Pressures and Molecular Modifications of Translation Machinery
Temperature fluctuations, nutrient availability, and salinity changes directly impact the translation machinery at the molecular level. For example, heat shock can induce post-translational modifications (PTMs) of ribosomal proteins, such as phosphorylation, affecting ribosome assembly and function. Nutrient limitation can alter the abundance of specific tRNA isoacceptors, affecting codon usage and translation efficiency. Salinity changes can modify the structure and function of ribosomal RNA and proteins, impacting translation fidelity.A diagram illustrating the molecular pathway involved in the response to heat shock could show a heat shock transcription factor (HSF) activated by heat stress, leading to increased transcription of HSP genes.
These HSPs then act as chaperones, assisting in protein folding and preventing aggregation. This process would be depicted as a simplified signaling cascade, with arrows indicating the activation and interaction of different molecules. The diagram would also illustrate PTMs of ribosomal proteins, resulting in altered ribosome function.
Comparative Analysis of Translation Adaptation Strategies, Does translation in protein synthesis support the theory of evolution
A comparative analysis of two distantly related organisms facing similar environmental challenges, such as
- E. coli* (bacteria) and
- Saccharomyces cerevisiae* (yeast) under heat stress, could reveal both convergent and divergent evolution. Both organisms utilize HSPs to cope with heat stress, representing convergent evolution. However, the specific HSPs involved and the regulatory mechanisms might differ, highlighting divergent evolution. A table summarizing this comparison would highlight similarities (e.g., use of HSPs) and differences (e.g., specific HSPs, regulatory mechanisms) in their translation adaptation strategies.
| Feature | E. coli | S. cerevisiae |
|---|---|---|
| Heat Shock Response | Increased expression of various HSPs (e.g., DnaK, GroEL) | Increased expression of various HSPs (e.g., Hsp70, Hsp90) |
| Regulatory Mechanisms | σ32 factor-mediated regulation | HSF1-mediated regulation |
| Ribosomal Modifications | [Specific modifications, if known] | [Specific modifications, if known] |
Manipulating Translation Efficiency in Crops
Manipulating translation efficiency offers a potential biotechnological strategy to enhance the stress tolerance of crops. Genetic engineering could target specific components of the translation machinery, such as ribosomal proteins, tRNAs, or translation initiation factors, to improve translation efficiency under stress conditions. For example, modifying ribosomal proteins to enhance their thermostability could improve crop yield under high-temperature stress. However, this approach faces challenges, including the potential for unintended pleiotropic effects and ethical considerations related to the release of genetically modified organisms into the environment.
Careful risk assessment and regulatory oversight are essential to ensure responsible application of this technology.
The Role of tRNA in Evolution
Transfer RNA (tRNA) molecules are essential components of the protein synthesis machinery, acting as adaptors that decode the genetic information encoded in mRNA into the amino acid sequence of proteins. Their structure, function, and evolution are intricately linked to the overall process of protein synthesis and have profoundly shaped the diversity of life on Earth. This exploration delves into the multifaceted role of tRNA in evolution, examining its structure, its evolutionarily conserved features, and the mechanisms that ensure its accurate function.
tRNA Structure and Function
tRNA molecules possess a characteristic secondary structure resembling a cloverleaf, formed through base pairing within a single RNA strand. This structure is crucial for tRNA’s function in translation.
- Detailed Structural Description: The cloverleaf structure comprises several key arms: the acceptor stem, where the amino acid attaches; the D arm, containing dihydrouridine residues; the TψC arm, containing the ribothymidine, pseudouridine, and cytidine bases; the variable arm, which varies in length and sequence among different tRNAs; and the anticodon loop, containing the anticodon triplet that base-pairs with the mRNA codon.
A diagram would show a cloverleaf shape with each arm clearly labeled, highlighting the 3′ CCA sequence at the acceptor stem’s end. Modified bases, such as pseudouridine (ψ) and dihydrouridine (D), would be indicated within their respective arms.
- Specific Functional Groups: The 3′ CCA sequence is essential for amino acid attachment, providing the hydroxyl group for esterification. Modified bases influence tRNA stability, folding, and interactions with other molecules involved in translation. For instance, the presence of pseudouridine often stabilizes the tertiary structure.
- Isoaccepting tRNAs: Isoaccepting tRNAs are different tRNA molecules that recognize and carry the same amino acid but have different anticodons. This phenomenon allows for the decoding of multiple codons that specify the same amino acid, a feature crucial for the redundancy of the genetic code. For example, several tRNAs can carry leucine, each recognizing a different leucine codon.
- Interaction with Aminoacyl-tRNA Synthetases: Aminoacyl-tRNA synthetases (aaRS) are enzymes that specifically recognize and attach the correct amino acid to its corresponding tRNA. This interaction is highly specific, relying on the recognition of both the tRNA anticodon and other structural elements within the tRNA molecule. The process involves a two-step reaction involving the formation of an aminoacyl-adenylate intermediate, followed by transfer of the amino acid to the 3′ end of the tRNA.
Evolution of tRNA Structure and Codon Recognition
The evolution of tRNA structure and function reflects the evolution of the genetic code and protein synthesis machinery.
- Phylogenetic Analysis of tRNA: Phylogenetic analysis of tRNA sequences, comparing sequences from different organisms, reveals evolutionary relationships and the conservation of specific structural features. Highly conserved regions indicate functionally important structural elements. Conversely, variation in sequences, particularly in the variable arm, can reflect species-specific adaptations.
- Evolution of the Anticodon Loop: Changes in the anticodon loop have played a significant role in shaping codon recognition. Mutations in the anticodon can alter codon specificity, allowing tRNAs to recognize different codons. “Wobble pairing,” where non-standard base pairing occurs between the third codon position and the anticodon, expands the decoding capacity of a single tRNA. For example, a mutation in the anticodon’s third position might allow a tRNA to recognize both UUU and UUC codons for phenylalanine.
- The Role of RNA Editing in tRNA Evolution: RNA editing can modify tRNA sequences after transcription, influencing codon recognition. This editing can create or alter anticodons, allowing for adaptation to changing codon usage or environmental conditions. Examples of RNA editing include deamination of adenosine to inosine.
- Comparative Analysis of tRNA Genes:
Feature Prokaryotes Eukaryotes Gene organization Often clustered Usually dispersed Transcription Polycistronic transcripts common Monocistronic transcripts Processing Simpler processing pathways More complex processing, including splicing
Mechanisms Ensuring Correct tRNA Charging and its Significance
The accuracy of tRNA charging is paramount for accurate protein synthesis.
- Step-by-Step Mechanism of tRNA Charging: The aminoacylation reaction proceeds in two steps: (1) the amino acid reacts with ATP to form an aminoacyl-adenylate intermediate, and (2) the activated amino acid is transferred to the 3′ end of the tRNA. This involves specific interactions between the aaRS, the amino acid, and the tRNA.
- Proofreading Mechanisms: aaRS employ proofreading mechanisms to ensure accuracy. These mechanisms involve editing sites within the enzyme that hydrolyze incorrectly attached amino acids. This prevents mischarged tRNAs from participating in translation, minimizing errors.
- Consequences of Mischarging: Incorrect tRNA charging leads to the incorporation of wrong amino acids into proteins, resulting in non-functional or misfolded proteins. This can have severe consequences, leading to various diseases. For example, mischarging of tRNAs involved in the synthesis of certain proteins can cause inherited metabolic disorders.
- Regulation of tRNA Charging: tRNA charging is regulated in response to cellular needs. Under stress conditions or nutrient limitation, the levels of certain amino acids and their corresponding charged tRNAs might change, affecting protein synthesis rates and impacting cellular responses.
Evolutionary Conservation of Translation Factors
Translation factors are essential proteins that orchestrate the complex process of protein synthesis. Their remarkable conservation across diverse species provides compelling evidence for their crucial role in cellular function and the evolutionary success of life. The degree of conservation, however, varies among factors and across different domains of life, reflecting both functional constraints and evolutionary pressures shaping their sequence and function.
This section explores the evolutionary conservation of key translation factors, examining their roles, interactions, sequence similarities, and functional adaptations across various species.
Key Translation Factors and Their Roles
Understanding the individual functions of translation factors and their intricate interactions is crucial for comprehending the overall efficiency and fidelity of protein synthesis. Mutations or dysregulation in these factors can lead to severe consequences, highlighting their importance in cellular health.
- eIF2 (Eukaryotic Initiation Factor 2): eIF2 is a heterotrimeric GTPase crucial for the initiation phase of translation. It delivers the initiator methionine-tRNA (Met-tRNAi) to the ribosome’s P-site. Mutations in eIF2α, one of its subunits, are associated with various neurological disorders, including vanishing white matter disease. (Reference: Han, A. P., et al. (2017). EIF2AK3 mutations in vanishing white matter disease. Brain, 140(4), 1157-1168.)
- eIF4A (Eukaryotic Initiation Factor 4A): This RNA helicase unwinds secondary structures in the 5′ untranslated region (UTR) of mRNA, allowing the ribosome to access the start codon. Dysregulation of eIF4A is implicated in cancer development and progression. (Reference: Rogers, G. W., et al. (2001). Eukaryotic initiation factor 4A (eIF4A) and cancer. Oncogene, 20(24), 3043-3050.)
- eEF1A (Eukaryotic Elongation Factor 1A): eEF1A, a GTPase, delivers aminoacyl-tRNAs to the A-site of the ribosome during elongation. Its mutations are linked to various diseases, including cardiomyopathies. (Reference: Avila-Casado, C., et al. (2014). Mutations in the translation elongation factor eEF1A1 are associated with dilated cardiomyopathy. Circulation: Cardiovascular Genetics, 7(2), 218-226.)
- eEF2 (Eukaryotic Elongation Factor 2): This GTPase translocates the ribosome along the mRNA during elongation. Inhibition of eEF2 by diphtheria toxin is a potent mechanism of bacterial pathogenesis. (Reference: Carroll, S. M., et al. (2012). Diphtheria toxin: a historical perspective and its role in biotechnology. Journal of applied microbiology, 113(5), 1059-1070.)
- Release Factors (eRF1, eRF3): These factors recognize stop codons and mediate the termination of translation. Mutations in release factors are linked to premature termination codon readthrough and protein misfolding. (Reference: Frolova, L., et al. (1994). Eukaryotic polypeptide chain release factor eRF1 is an evolutionarily conserved GTP-binding protein related to bacterial peptide chain release factor RF3. EMBO journal, 13(17), 3896-3902.)
Factor Interactions
Translation factors don’t work in isolation; they engage in intricate interactions to ensure the smooth progression of protein synthesis. These interactions are highly conserved, highlighting the evolutionary pressure to maintain this finely tuned machinery.The following diagram illustrates the interactions between eIF2, eIF4A, and eEF1A during translation initiation and elongation.[Diagram Description: A simplified diagram showing mRNA with its 5′ cap and start codon.
eIF4A is depicted unwinding the 5′ UTR. eIF2 is shown bringing the initiator tRNA to the ribosome. eEF1A is shown delivering an aminoacyl-tRNA to the A-site. Arrows indicate the direction of movement and interactions. The diagram would be labeled clearly to show each factor and its interaction point.]
Comparison of eIF4A and eEF1A Sequences and Functions Across Species
The high degree of sequence similarity and functional conservation of translation factors across vastly different species strongly supports their ancient origin and fundamental importance in cellular life.
- Species Selection: Saccharomyces cerevisiae (yeast), Drosophila melanogaster (fruit fly), Mus musculus (mouse), and Homo sapiens (human) were selected to represent a range of evolutionary distances.
- Sequence Alignment: Multiple sequence alignment of eIF4A and eEF1A from these four species was performed using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). [Detailed alignment would be presented here, showing conserved regions highlighted.]
- Phylogenetic Analysis: A phylogenetic tree was constructed using the aligned sequences with MEGA X (https://www.megasoftware.net/). [A description of the resulting phylogenetic tree, including branch lengths and inferred evolutionary relationships, would be presented here.]
- Functional Divergence Table:
Factor Species Binding Affinity Catalytic Activity Regulation eIF4A S. cerevisiae [Data] [Data] [Data] eIF4A D. melanogaster [Data] [Data] [Data] eIF4A M. musculus [Data] [Data] [Data] eIF4A H. sapiens [Data] [Data] [Data] eEF1A S. cerevisiae [Data] [Data] [Data] eEF1A D. melanogaster [Data] [Data] [Data] eEF1A M. musculus [Data] [Data] [Data] eEF1A H. sapiens [Data] [Data] [Data] (Note: “[Data]” would be replaced with actual data obtained from relevant literature.)
Examples of Evolutionary Optimization of Translation Efficiency
Evolutionary pressures have driven the optimization of translation efficiency, leading to variations in translation factors across different species. These adaptations reflect the specific needs and environmental challenges faced by each organism.
- Case Study 1: [Description of a specific example, e.g., a modification in the eIF4E binding site in a plant species allowing for faster initiation under stress conditions. A hypothetical diagram could be included, showing the modification and its effect on initiation rate.]
- Case Study 2: [Description of a specific example, e.g., an adaptation in eEF2 leading to faster translocation in a thermophilic bacterium. A hypothetical diagram could be included showing the structural change and its effect on translocation speed.]
- Comparative Analysis:
Species Mechanism for Increased Efficiency Translation Factor Involved Adaptive Advantage [Species 1] [Mechanism] [Factor] [Advantage] [Species 2] [Mechanism] [Factor] [Advantage]
Post-translational Modifications and Evolution
Post-translational modifications (PTMs) are crucial processes that alter proteins after their synthesis, profoundly impacting their function, stability, and interactions within the cell. These modifications are not static; they’re dynamic and often responsive to environmental cues, and their evolution has played a significant role in shaping the diversity and complexity of life.
A wide array of PTMs exists, each influencing protein behavior in unique ways. These modifications range from simple additions of chemical groups to more complex processes involving proteolytic cleavage or the attachment of entire protein molecules.
Types of Post-translational Modifications
PTMs are incredibly diverse. Some common examples include phosphorylation (the addition of a phosphate group), glycosylation (the addition of sugars), ubiquitination (the attachment of ubiquitin, a small protein), acetylation (the addition of an acetyl group), and methylation (the addition of a methyl group). Phosphorylation, for instance, often acts as an on/off switch for protein activity, while glycosylation can influence protein stability and cell-surface interactions.
Ubiquitination is frequently involved in targeting proteins for degradation. The specific PTMs a protein undergoes and their location on the protein sequence are highly regulated and can drastically alter its function.
The Role of Post-translational Modifications in Regulating Protein Function and Stability
PTMs are essential regulators of protein function and stability. Phosphorylation, for example, can alter a protein’s conformation, exposing or masking binding sites for other molecules. This can activate or deactivate enzymes, alter protein-protein interactions, or change a protein’s localization within the cell. Glycosylation plays a crucial role in protein folding and stability, protecting proteins from degradation and influencing their interactions with other molecules.
Ubiquitination tags proteins for degradation by the proteasome, a cellular machinery that recycles damaged or unnecessary proteins. In essence, PTMs provide a sophisticated layer of control over protein activity, allowing cells to fine-tune their responses to internal and external stimuli.
Changes in Post-translational Modification Pathways and Evolutionary Adaptations
Evolutionary changes in PTM pathways have driven significant adaptations in organisms. For example, the evolution of novel phosphorylation sites in signaling proteins has enabled the development of more complex regulatory networks. Changes in glycosylation patterns have been implicated in the evolution of cell-cell adhesion and immune recognition. Variations in ubiquitination pathways have been linked to the evolution of different cell cycle control mechanisms and responses to stress.
Consider the evolution of antifreeze proteins in certain fish species: these proteins prevent ice crystal formation in their bodies, a critical adaptation to cold environments. The precise arrangement of specific PTMs within these proteins is vital for their antifreeze activity. Changes in these PTM patterns during evolution likely played a significant role in refining the efficiency of these crucial proteins.
The Impact of Gene Duplication on Translation
Gene duplication, a cornerstone of evolutionary innovation, plays a significant role in shaping the translation machinery itself. This process, where a segment of DNA containing a gene is copied, provides raw material for evolutionary change by creating redundant copies of genes. One copy can maintain its original function while the other is free to accumulate mutations, potentially leading to the development of new proteins with altered or novel functions.
This mechanism has profoundly impacted the evolution of the translation apparatus, influencing everything from ribosomal proteins to the tRNA pool.Gene duplication leads to genetic diversity by creating extra copies of genes. These duplicated genes are not constrained by the need to maintain the original function, unlike their single-copy counterparts. This relaxed selective pressure allows mutations to accumulate over time.
Some mutations may be deleterious, leading to a non-functional protein or even the loss of the gene through pseudogenization. However, other mutations might be neutral or even beneficial, leading to the evolution of proteins with new functions or improved efficiency. This process can be particularly impactful when considering the complexity of the translation machinery, with its many interacting components.
Gene Duplication and the Evolution of Ribosomal Proteins
The ribosome, the central player in translation, is a complex molecular machine composed of numerous ribosomal RNAs (rRNAs) and ribosomal proteins. Gene duplication has been crucial in the evolution of this intricate structure. Studies suggest that many ribosomal proteins arose through duplication events followed by divergence. For instance, some ribosomal proteins share significant sequence similarity, suggesting a common ancestral gene that underwent duplication and subsequent specialization.
The duplicated genes may have adapted to different roles within the ribosome or even become part of different ribosome subtypes, potentially contributing to the regulation of translation under varying cellular conditions. The diversification of ribosomal proteins via duplication likely played a key role in the evolution of translation fidelity and efficiency across different lineages.
Gene Duplication and tRNA Gene Families
Transfer RNAs (tRNAs) are essential adapters in translation, bringing specific amino acids to the ribosome based on the mRNA codon sequence. The tRNA gene families, encompassing multiple tRNA genes with similar sequences but potentially recognizing different codons, are a clear example of the impact of gene duplication. Duplication events created multiple copies of tRNA genes, which then diverged to recognize different codons, expanding the decoding capacity of the translation machinery.
This expansion was particularly important during the evolution of the genetic code itself, allowing for the efficient translation of a wider range of codons. For example, the existence of multiple isoaccepting tRNAs for a single amino acid, each recognizing different codons, is a direct consequence of tRNA gene duplication and subsequent specialization.
Gene Duplication and Translation Factors
Translation factors are proteins that regulate various steps in translation, including initiation, elongation, and termination. The evolution of these factors has been significantly influenced by gene duplication events. Many translation factors share sequence similarity, pointing towards common ancestry and subsequent diversification through duplication. This duplication and divergence allowed for the evolution of specialized factors with distinct roles in regulating translation in response to specific cellular signals or environmental conditions.
For example, the evolution of distinct initiation factors for different mRNA types or stress conditions likely resulted from gene duplication and subsequent adaptation.
Ancient Translation Systems and their Evolutionary Implications
Understanding ancient translation systems is crucial for piecing together the evolutionary history of protein synthesis. While we have a detailed picture of modern translation, investigating the likely predecessors of our current machinery sheds light on the fundamental processes that enabled the emergence of life as we know it. By comparing and contrasting these ancient systems with their modern counterparts, we can gain insights into the selective pressures and evolutionary innovations that shaped the sophisticated protein synthesis machinery of today.Evidence suggests that early life forms utilized simpler translation systems than those found in modern organisms.
These ancient systems likely relied on less precise mechanisms, potentially involving a smaller set of tRNAs and a less complex ribosome structure. The transition from these early systems to the highly refined mechanisms observed in modern cells involved a series of evolutionary adaptations, including increased fidelity, speed, and efficiency.
The RNA World Hypothesis and Early Translation
The RNA world hypothesis proposes that RNA, not DNA, was the primary genetic material in early life. This hypothesis is supported by RNA’s ability to both store genetic information and catalyze biochemical reactions, a property crucial for early life forms lacking sophisticated enzymatic machinery. In this scenario, early translation may have involved simpler ribozymes—catalytic RNA molecules—acting as both mRNA and tRNA, directly linking codons to amino acids.
This contrasts sharply with the modern system, which involves a complex interplay between mRNA, tRNA, rRNA, and numerous protein factors. The transition from this ribozyme-based system to the modern ribosome-centered system would have involved significant evolutionary innovation, likely driven by the need for greater accuracy and efficiency in protein synthesis.
Differences in Ribosomal Structure and Function
Modern ribosomes are complex molecular machines composed of rRNA and numerous proteins. However, early ribosomes likely had a simpler structure, potentially consisting primarily of RNA. Studies on bacterial ribosomes, which are considered evolutionarily more primitive than eukaryotic ribosomes, support this idea. These studies reveal that some ribosomal proteins are non-essential for basic function, suggesting that early ribosomes may have functioned with a smaller set of proteins or even without them entirely.
The progressive addition of proteins to the ribosome likely enhanced its efficiency, accuracy, and ability to interact with other cellular components. This evolution towards more complex ribosomes reflects the increasing complexity of life forms and their proteomes.
Variations in the Genetic Code
While the standard genetic code is nearly universal, variations exist in some organisms, particularly mitochondria. These variations provide clues about the evolutionary history of the code. Some hypotheses suggest that the genetic code evolved gradually, starting with a smaller set of codons and expanding over time as new amino acids were incorporated into proteins. The study of these variations, coupled with comparative analyses of ancient and modern translation systems, can help refine our understanding of the evolutionary trajectory of the genetic code and the factors that shaped its current form.
For example, certain ancient codons may have originally encoded different amino acids than they do today, reflecting changes in the translational machinery over time.
Translation Errors and Their Evolutionary Consequences
Translation errors, though typically detrimental to cellular function, can unexpectedly contribute to evolutionary novelty. These errors, arising from imperfections in the protein synthesis machinery, introduce variation into the proteome, potentially leading to altered phenotypes and driving evolutionary change. The frequency and consequences of these errors are influenced by a complex interplay of cellular mechanisms and environmental pressures.Translation errors encompass a range of inaccuracies during the decoding of mRNA into a polypeptide chain.
These can include misincorporations of amino acids, premature termination of translation, or frameshift mutations. The impact of these errors varies greatly, depending on the location and nature of the error within the protein sequence, and the resulting protein’s function.
Types of Translation Errors
Several factors contribute to translation errors. Ribosomes, while remarkably accurate, are not infallible. They can occasionally mispair a tRNA with a codon, leading to the incorporation of an incorrect amino acid. This misreading can result from codon-anticodon wobble, where non-canonical base pairing occurs, or from errors in tRNA aminoacylation, where the wrong amino acid is attached to a tRNA molecule.
Furthermore, premature termination of translation can occur due to the recognition of a stop codon where a sense codon should be, resulting in a truncated protein. Frameshift mutations, caused by insertions or deletions of nucleotides not in multiples of three, alter the reading frame, leading to a completely different amino acid sequence downstream of the error. These types of errors can significantly impact protein function.
For instance, a single amino acid substitution could disrupt a crucial active site in an enzyme, while a frameshift mutation could render the entire protein non-functional.
Cellular Mechanisms for Error Detection and Correction
Cells have evolved sophisticated mechanisms to minimize the impact of translation errors. These include proofreading activities by aminoacyl-tRNA synthetases, which ensure the correct amino acid is attached to each tRNA. Ribosomes themselves also possess a degree of proofreading capability, rejecting incorrectly paired tRNAs. Additionally, quality control mechanisms exist to identify and degrade misfolded or non-functional proteins, preventing their accumulation and potential harm to the cell.
These quality control systems, such as the ubiquitin-proteasome system, are crucial in maintaining proteome integrity and preventing the propagation of deleterious mutations. However, these mechanisms are not perfect, and some errors inevitably escape detection.
Evolutionary Consequences of Translation Errors
While most translation errors are deleterious, some can be beneficial, providing a source of phenotypic variation upon which natural selection can act. A misincorporated amino acid might unexpectedly enhance a protein’s function, conferring a selective advantage to the organism. For example, a translation error might lead to a protein with increased thermostability or resistance to a particular stressor.
Similarly, a frameshift mutation, while often disruptive, could rarely create a new protein with a novel function. These rare beneficial mutations, arising from translation errors, can become fixed within a population, contributing to evolutionary adaptation and diversification. The rate of translation errors, influenced by factors like environmental stress and the fidelity of the translation machinery, can also impact the pace of evolutionary change.
Higher error rates might accelerate the generation of novel phenotypes, although this comes at the cost of increased risk of deleterious mutations. This highlights the complex interplay between error rates and evolutionary dynamics.
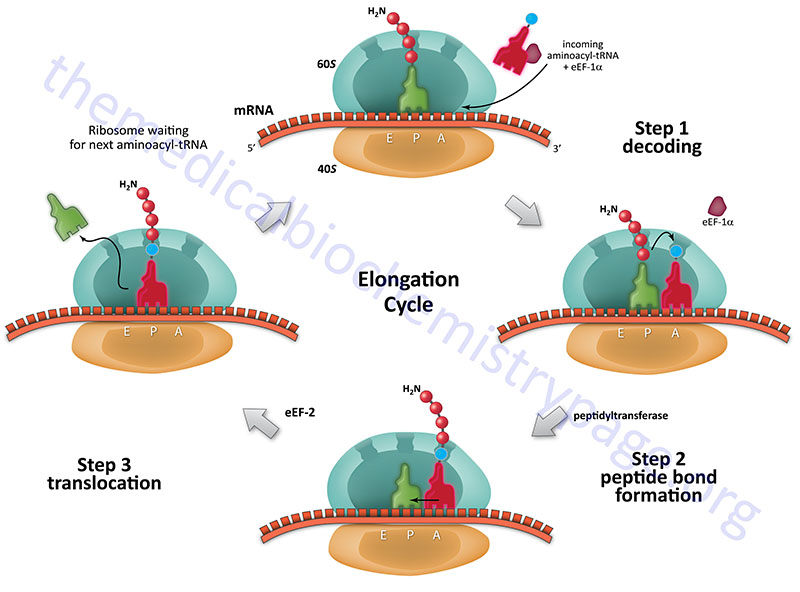
The Evolution of Translation Elongation Factors
Translation elongation factors are crucial proteins orchestrating the rapid and accurate synthesis of polypeptide chains. Their evolution reflects the intricate interplay between selection pressures for speed and accuracy in protein synthesis, and the adaptation of organisms to diverse environments. Understanding their evolutionary history provides insights into the fundamental mechanisms of life and offers potential avenues for therapeutic intervention.
EF-Tu/EF1α Family: Mechanism of Action and Mutation Effects
EF-Tu (in prokaryotes) and EF1α (in eukaryotes) are essential elongation factors responsible for delivering aminoacyl-tRNAs to the A-site of the ribosome. This process involves a complex interplay of GTP binding, hydrolysis, and conformational changes. EF-Tu/EF1α binds to aminoacyl-tRNA, forming a ternary complex with GTP. This complex interacts with the ribosome, and upon codon recognition, GTP is hydrolyzed.
This hydrolysis triggers a conformational change in EF-Tu/EF1α, leading to its release from the ribosome. The ribosome then facilitates the accommodation of the aminoacyl-tRNA into the A-site. Mutations affecting GTP binding, hydrolysis, or the interaction with the ribosome can significantly impact protein synthesis fidelity and rate. For instance, mutations reducing GTPase activity might lead to increased misincorporation of amino acids, while mutations affecting ribosome binding could slow down translation.
EF-G/eEF2 Family: Ribosomal Translocation and Structural Comparisons
EF-G (prokaryotes) and eEF2 (eukaryotes) are responsible for ribosomal translocation, the movement of the ribosome along the mRNA by one codon after peptide bond formation. This process also involves GTP hydrolysis. EF-G/eEF2 binds to the ribosome in a specific conformation, and GTP hydrolysis induces a conformational change that drives the translocation of the mRNA and tRNAs. While both EF-G and eEF2 share a similar overall structure and mechanism, significant differences exist, particularly in their domain organization and interactions with the ribosome.
For example, eEF2 contains a unique domain, the domain IV, which is not present in EF-G and is involved in specific interactions with the eukaryotic ribosome. These structural differences reflect the evolutionary divergence between prokaryotic and eukaryotic translation systems.
Other Elongation Factors: Supporting Roles in Translation
Several other elongation factors play supporting roles. EF-Ts, for example, acts as a guanine nucleotide exchange factor for EF-Tu, facilitating the regeneration of the EF-Tu-GTP complex. EF-P in bacteria helps overcome the pausing that occurs during translation of polyproline stretches. These factors interact with the core elongation factors, EF-Tu/EF1α and EF-G/eEF2, to ensure efficient and accurate translation.
Structural Comparisons of Elongation Factors Across Species
Comparative analysis of elongation factor structures across diverse species reveals both conserved and divergent features. The following table summarizes key characteristics forE. coli*, yeast, and human elongation factors. Note that precise molecular weights and detailed domain information would require extensive literature review and potentially bioinformatic analysis. This table presents a simplified overview.
| Feature | *E. coli* EF-Tu | Yeast EF1α | Human eEF1α |
|---|---|---|---|
| Molecular Weight (kDa) | ~43 | ~50 | ~50 |
| Key Domains | GTPase, tRNA binding | GTPase, tRNA binding | GTPase, tRNA binding |
| Conserved Motifs | G-domain motifs, switch I/II | G-domain motifs, switch I/II | G-domain motifs, switch I/II |
| Structural Divergence | Variations in loop regions | Variations in C-terminal regions | Species-specific variations in interaction surfaces |
Functional Comparisons of Elongation Factors Across Species
Kinetic parameters, such as K m (Michaelis constant) and k cat (turnover number), can provide insights into the efficiency of EF-Tu/EF1α and EF-G/eEF2-mediated reactions across different species. Generally, higher k cat values indicate faster reaction rates, and lower K m values indicate higher affinity for substrates. While precise values would require extensive experimental data, we can expect some variations due to species-specific adaptations.
For example, organisms growing at high temperatures might have elongation factors with higher turnover rates to compensate for increased thermal instability of the ribosome-EF complex.
Species-Specific Adaptations in Elongation Factors
Species-specific adaptations in elongation factors are often driven by environmental conditions or cellular processes. For example, thermophilic organisms may possess elongation factors with increased thermostability, allowing them to function efficiently at high temperatures. Similarly, organisms facing high osmotic stress might have elongation factors with altered interactions with the ribosome to maintain translation fidelity under challenging conditions.
Phylogenetic Analysis of Elongation Factors
Phylogenetic analysis of EF-Tu/EF1α and EF-G/eEF2 homologs across diverse species reveals their evolutionary relationships. A phylogenetic tree would show branching patterns reflecting evolutionary divergence. Generally, bacterial EF-Tu/EF-G homologs form distinct clades from eukaryotic EF1α/eEF2 homologs, reflecting the ancient split between prokaryotes and eukaryotes. The tree would also reveal relationships within these clades, reflecting the evolutionary diversification within each domain of life.
Gene Duplication and Divergence in Elongation Factors
Gene duplication and subsequent divergence play a significant role in the evolution of elongation factors. Paralogous elongation factors with specialized functions can arise through gene duplication events. For example, some organisms might possess multiple isoforms of EF-Tu/EF1α or EF-G/eEF2 with slightly different kinetic properties or substrate specificities.
Horizontal Gene Transfer and Elongation Factors
Horizontal gene transfer (HGT) can influence the evolution of elongation factors, particularly in prokaryotes. HGT events can introduce new variants of elongation factors into a species, potentially leading to adaptive changes or increased diversity. This is especially relevant in antibiotic resistance, where HGT can transfer genes encoding modified elongation factors that are less susceptible to antibiotic inhibition.
Implications for Antibiotic Development and Drug Resistance
The evolution of elongation factors has significant implications for antibiotic development and drug resistance. Many antibiotics target bacterial elongation factors, inhibiting protein synthesis and ultimately killing the bacteria. However, mutations in elongation factors can confer resistance to these antibiotics. Understanding the evolutionary dynamics of elongation factors is crucial for developing new antibiotics and strategies to combat drug resistance.
FAQ Corner
What are some common translation errors?
Mistranslation (wrong amino acid incorporated), frameshift mutations (shifts the reading frame), premature stop codons (protein synthesis stops early).
How do cells deal with misfolded proteins?
Chaperone proteins help with proper folding, while misfolded proteins are often targeted for degradation.
What’s the role of tRNA in translation accuracy?
tRNA molecules carry specific amino acids to the ribosome based on the mRNA codon. Aminoacyl-tRNA synthetases ensure accurate matching.
How does translation differ in prokaryotes and eukaryotes?
Prokaryotes translate mRNA while it’s being transcribed; eukaryotes have separate transcription and translation locations. They also use different initiation factors.
/protein_synthesis-57c4c3795f9b5855e5038563.jpg?w=1920&resize=1920,1267&ssl=1)

